Table 1.
Intermolecular Aminofluorination Reactions – the Scope of Alkenes and Amines.
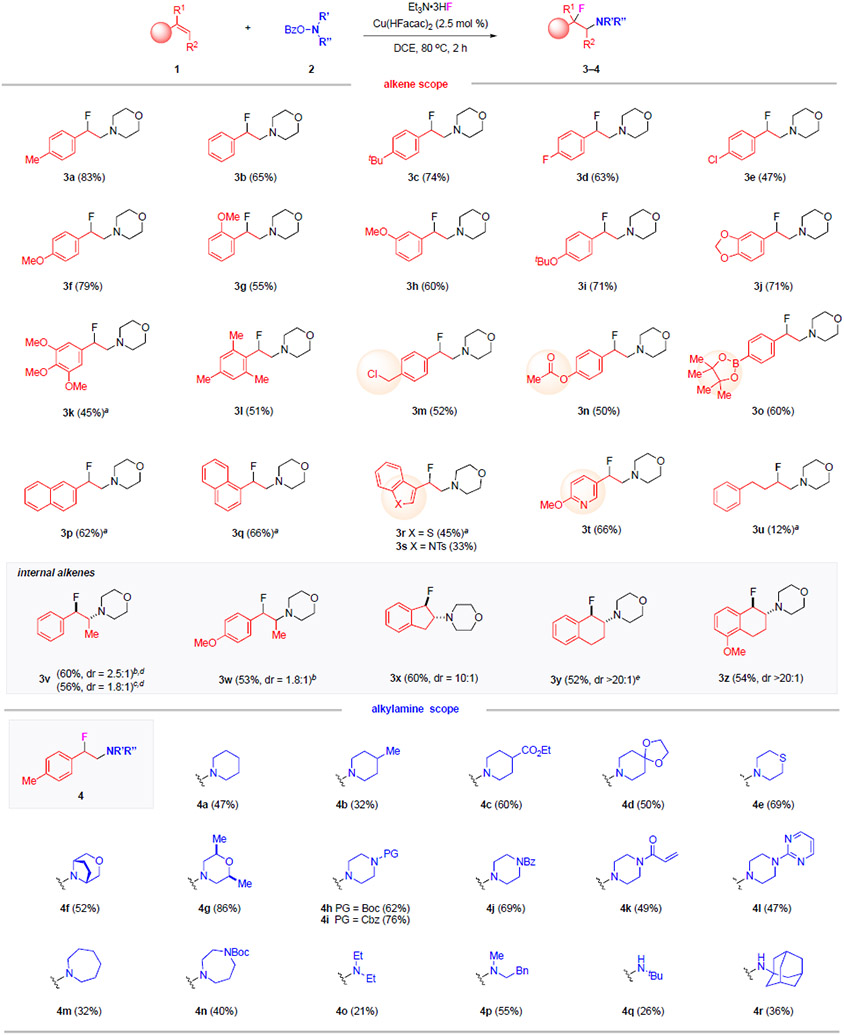
|
Reaction conditions: 1 (2.0 equiv), 2 (1.0 equiv), Et3N•3HF (10 equiv), Cu(HFacac)2 (2.5 mol %), DCE (1.0 mL), 80 °C, 2 h. Isolated yields shown. dr determined by 1H-NMR analysis of the crude mixtures. Major isomer shown. aIPrCuCl (5.0 mol %) used. bFrom E-alkene. cFrom Z-alkene. dRelative stereochemistry of the major diastereomer determined by X-ray analysis. eCH3(CH2)10C(O)O–NR’R” used.
