This cohort study investigates the association of polygenic risk with visual field progression in early glaucoma.
Key Points
Question
What is the association of polygenic risk with the progression of vision loss in early glaucoma?
Findings
In a longitudinal cohort study of individuals with suspected glaucoma and individuals with early glaucoma, high glaucoma polygenic risk was associated with a more rapid rate of glaucomatous visual field worsening and a faster rate of nerve fiber layer thinning.
Meaning
Glaucoma polygenic risk may stratify risk of glaucoma worsening in early glaucoma and could be used to identify high-risk patients with glaucoma.
Abstract
Importance
Irreversible vision loss from primary open-angle glaucoma (POAG) can be prevented through timely diagnosis and treatment, although definitive diagnosis can be difficult in early-stage disease. As a consequence, large numbers of individuals with suspected glaucoma require regular monitoring, even though many of these may never develop disease and other high-risk individuals with suspected glaucoma may have delayed or inadequate treatment. POAG is one of the most heritable common diseases, and this provides an opportunity to use genetic instruments in risk-stratified screening, diagnosis, and treatment of early glaucoma.
Objective
To assess the association of glaucoma polygenic risk with glaucoma progression in early-stage disease.
Design, Setting, and Participants
This cohort study used clinical and genetic data obtained from a longitudinal cohort study, Progression Risk of Glaucoma: Relevant SNPs With Significant Association (PROGRESSA). Participants of European ancestry with characteristic optic nerve head changes suggestive of glaucoma were included. Data were collected between February 2012 and June 2020. Analysis took place between July 2020 and April 2022.
Main Outcomes and Measures
The association of a glaucoma polygenic risk score (PRS) (2673 uncorrelated variants) with rate of peripapillary retinal nerve fiber layer thinning on optical coherence tomography and progression of visual field loss on 24-2 Humphrey visual fields.
Results
A total of 1777 eyes from 896 individuals had sufficient data for structural progression analyses and 1563 eyes from 808 individuals for functional progression analyses. The mean (SD) age was 62.1 (9.9) years, 488 (44%) were male, and 1087 of 1103 individuals (98.5%) had European ancestry. An ancestrally matched normative population cohort (n = 17 642) was used for PRS reference. Individuals in the top 5% PRS risk group were at a higher risk of visual field progression compared with the remaining 95% after 5 years (hazard ratio, 1.5; 95% CI, 1.13-1.97; P = .005). Conversely, those in the bottom 20% PRS risk group were at a lower risk of visual field progression compared with an intermediate risk group over 3 years (hazard ratio, 0.52; 95% CI, 0.28-0.96; P = .04).
Conclusions and Relevance
In this study, high polygenic risk was associated with more rapid structural and functional progression in early POAG, despite more intensive treatment. A PRS may serve as a valuable adjunct to identify individuals who stand to benefit the most from more frequent surveillance and earlier or more intensive treatment.
Introduction
Polygenic risk scores (PRSs) are a probabilistic summary of an individual’s genetic risk of a disease or trait and are increasingly recognized as a tool in disease risk prediction and phenotyping.1 In the context of glaucoma, PRSs have been shown to effectively stratify risk of glaucoma, age at diagnosis, and the rate of optical coherence tomography (OCT) thinning in early manifest glaucoma cases.2,3,4,5,6 Peripapillary retinal nerve fiber layer (pRNFL) thinning (measured by serial OCT scans) is a marker of glaucoma worsening and often proceeds visual field loss.7 A key unresolved question is whether PRS can also predict the progressive visual field loss and pRNFL thinning characteristic of glaucoma, which in combination with other clinical risk factors could help identify individuals who would benefit from more frequent surveillance and earlier or more intensive treatment. A group in which this could be applied are individuals suspected of having glaucoma, where diagnostic uncertainty leads to the monitoring of large numbers of individuals, many of whom are low risk and ultimately never develop the disease, while other high-risk individuals may have delayed or inadequate treatment prior to demonstrating severe visual field loss. Using a prospective longitudinal study of individuals with early primary open-angle glaucoma, here we investigated the association of polygenic risk with visual field progression in early glaucoma.
Methods
Glaucoma Progression Data Set
We sampled 1103 genotyped participants from the PROGRESSA (Progression Risk of Glaucoma: Relevant SNPs With Significant Association) study. Detailed inclusion and assessment criteria are described in the eAppendix in the Supplement, following relevant reporting guidelines (Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] reporting guideline). Data were collected between February 2012 and June 2020.
Genotyping, Imputation, and PRS Derivation
PROGRESSA and QSkin (a prospective cohort aged 40 to 69 years, randomly sampled from Queensland, Australia)8 samples were genotyped on Omni 1M, OmniExpress, or HumanCoreExome arrays (Illumina). Imputation and derivation of the glaucoma PRS is described in detail elsewhere2 and is summarized in the eAppendix in the Supplement.
Statistical Analysis
For visual field worsening, a mixed-effect Cox proportional hazards regression model with a random intercept per patient (accounting for intereye correlation) was used, adjusting for baseline covariates of race, age, sex (self-reported), and visual field mean deviation. Visual field survival curves used in the plots were computed using 1 eye per individual (earlier progressing) to maintain visual and statistical validity. Linear mixed-effect models were used to assess the OCT pRNFL rate of thinning, with adjustment for OCT device type, and a random intercept per patient. Statistical analyses were performed using R version 4.0.2 (R Foundation), with mixed-effect models fitted using lme4 (R version 1.1.23), and coxme (R version 2.2.16) packages. P values were 2-sided and adjusted by Bonferroni correction when multiple comparisons were performed. The significance threshold was .05. Analysis took place between July 2020 and April 2022.
Results
A total of 1777 eyes from 896 individuals had sufficient data for structural progression analyses and 1563 eyes from 808 individuals for functional progression analyses. The mean (SD) age was 62.1 (9.9) years, 488 (44%) were male. Overall, 1087 of 1103 individuals (98.5%) self-reported having European ancestry, and the genetic ancestry reported more than 99% European. An ancestrally matched normative population cohort (n = 17 642) was used for PRS reference. To assess the association of polygenic risk with glaucoma progression, we used a multitrait glaucoma PRS2 in a longitudinal cohort of individuals suspected of having glaucoma and individuals with early-manifest glaucoma. High polygenic risk was defined as the top 5% of a normative population distribution, which was set using an ancestrally matched normative population cohort (QSkin) and which captured 11.97% of the PROGRESSA cohort (Figure 1A). This threshold has been shown to confer a risk of developing glaucoma similar to that seen for carriers of the most common single-gene high-penetrance primary open-angle glaucoma variant (MYOC p.Gln368Ter).6
Figure 1. Visual Field Worsening in Patients With Early-Manifest Glaucoma and Individuals With Suspected Glaucoma With High Polygenic Risk.
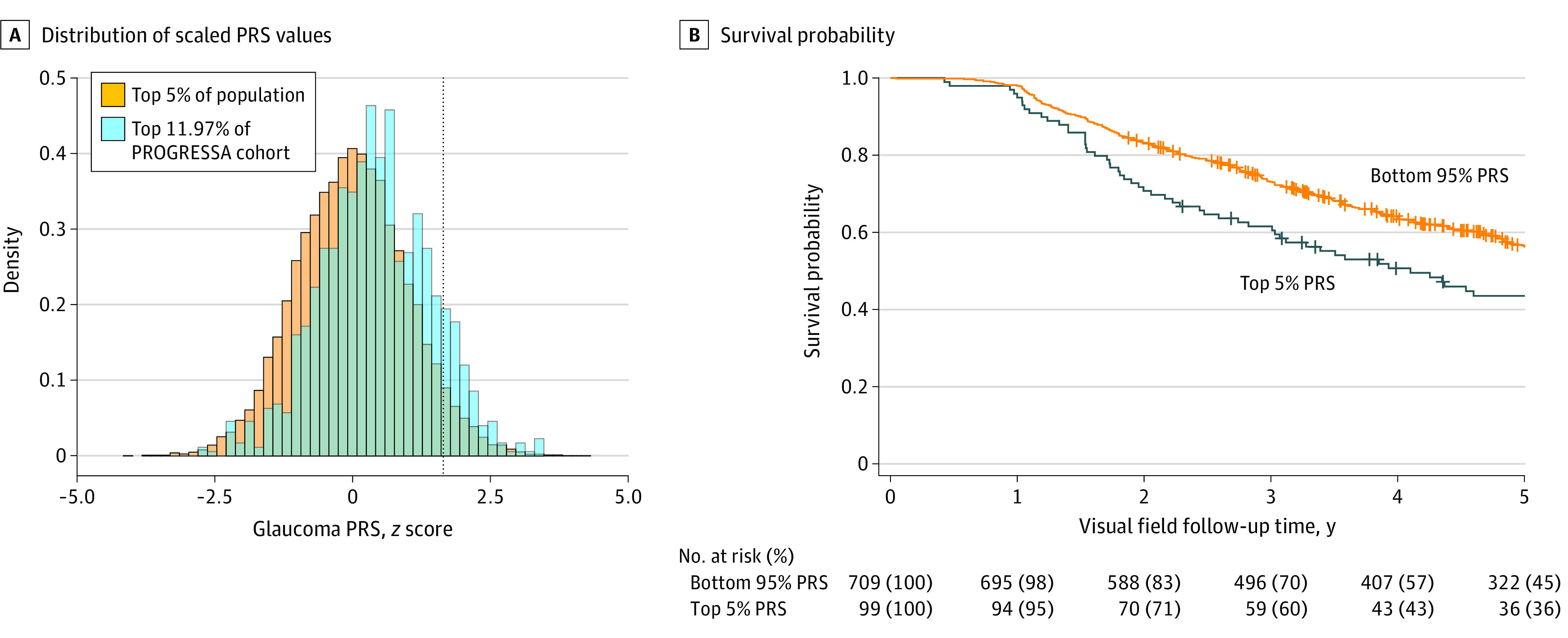
A, Distribution of scaled polygenic risk score (PRS) values (z scores) for the Progression Risk of Glaucoma: Relevant SNPs With Significant Association (PROGRESSA) cohort of patients with early-manifest glaucoma and those with suspected glaucoma, compared with an unselected control population. B, Individuals in the top 5% of the population PRS distribution showed accelerated visual field loss compared with the bottom 95% PRS group.
Both structural and functional glaucoma progression outcomes were compared, as both are known to be important in early glaucoma diagnosis and treatment.9 Longitudinal visual field worsening was the primary outcome in this cohort (Figure 1B). The mean visual field mean (SD) deviation was −1.1 (2.0) dB, which was not different between PRS groups at baseline (mean difference, −0.40 db; 95% CI, 0.06 to −0.85; P = .10). Although receiving higher treatment intensity (mean [SD], 1.57 [1.48] vs 1.15 [1.52] drops, selective laser trabeculoplasty procedures and/or trabeculectomies; P < .001), the top 5% PRS group had a greater likelihood of visual field worsening compared with the bottom 95% PRS group (hazard ratio, 1.5; 95% CI, 1.13-1.97; P = .005, Cox proportional hazards regression) (Table). We validated this finding using the Collaborative Initial Glaucoma Treatment Study progression criteria as the outcome with consistent results (eAppendix in the Supplement).
Table. Characteristics of Individuals With Early-Manifest Glaucoma or Individuals With Suspected Glaucoma Stratified by Genetic Risk.
| Characteristic | PRS, No. (%) | P value (top 5% PRS vs bottom 95%)a | |
|---|---|---|---|
| Top 5% | Bottom 95% | ||
| No. (%) | 132 (11.97) | 971 (88.03) | NA |
| Male | 55 (41.7) | 433 (44.6) | >.99 |
| Female | 77 (58.3) | 538 (55.4) | |
| Age at enrollment, mean (SD), y | 61.48 (9.58) | 62.14 (9.91) | >.99 |
| Family history of glaucoma | 74 (56.1) | 361 (37.2) | <.001 |
| Maximum recorded IOP, mean (SD), mm Hg | 22.40 (5.41) | 21.85 (6.44) | .44 |
| No. of glaucoma drops, SLT, or trabeculectomies, mean (SD) | 1.57 (1.48) | 1.15 (1.52) | <.001 |
| Rate of pRNFL thinning in the faster progressing quadrant, μm/y | −1.64 (1.40) | −1.36 (1.30) | .01 |
| Proportion of fast pRNFL progressors, >1 μm/y, % | 47.6 | 39.6 | .02b |
Abbreviations: IOP, intraocular pressure; NA, not applicable; pRNFL, peripapillary retinal nerve fiber layer; PRS, polygenic risk score; SLT, selective laser trabeculoplasty.
P values represent Bonferroni-corrected post hoc pairwise comparisons between the top 5% and bottom 95% of the population PRS distribution, except for the pRNFL outcomes. Maximum recorded IOP was obtained at any points during follow-up (not exclusively pretreatment IOP).
Results of a binomial model: odds ratio, 1.5; 95% CI, 1.09-2.2; P = .02.
We also investigated longitudinal structural progression represented by the rate of OCT-derived pRNFL thinning in the fastest-progressing quadrant (mean [SD] follow-up, 4.7 [1.7] years; Table). Individuals in the top 5% PRS group had a faster rate of pRNFL thinning compared with the bottom 95% PRS group (mean [SD], −1.64 [1.40] vs −1.36 [1.30] μm/y; 95% CI, −0.05 to −0.49 μm/year; P = .01) after adjustment for age, self-reported sex, and OCT device. We also defined a binary outcome of fast pRNFL progression as a rate of pRNFL thinning more than 1 μm per year, which is 5 times faster than a previously reported rate of age-related pRNFL thinning.10 Despite higher treatment intensity, individuals in the top 5% PRS group had a greater likelihood of fast pRNFL progression compared with the bottom 95% PRS group (odds ratio, 1.5; 95% CI, 1.1-2.2; P = .02).
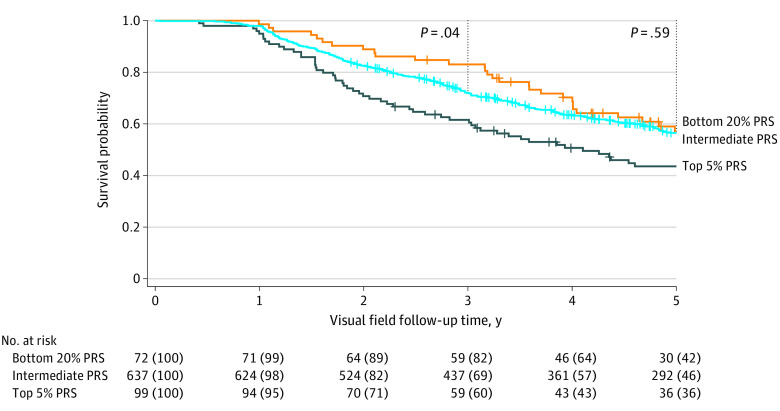
We then investigated progression in a low–genetic risk group, defined as the bottom 20% of the normative population PRS distribution. Due to the right-shifted nature of this cohort’s PRS distribution (Figure 1), we selected a normative population threshold that captured an approximately similar number of individuals as the top 5%: 72 individuals in the bottom 20% vs 99 in the top 5% group. The bottom 20% PRS group appeared to have slower visual field worsening events compared with the intermediate 75% group (Figure 2); however, this was only statistically significant up to the 3-year follow-up (hazard ratio, 0.52; 95% CI, 0.28-0.96; P = .04). Similarly, the bottom 20% PRS group had a slower rate of pRNFL progression compared with the intermediate 75% group by 0.27 μm per year (95% CI, 0.02-0.05; P = .04), suggesting a slower course of disease progression.
Figure 2. Visual Field Worsening in Patients With Early-Manifest Glaucoma and Individuals With Suspected Glaucoma With Low Polygenic Risk.
Individuals in the high-risk distribution (top 5% of the population polygenic risk score [PRS]) showed a more rapid visual field worsening, whereas the low-risk distribution (bottom 20% of the population PRS) had slower worsening compared with the intermediate 75% group at 3 years (hazard ratio, 0.52; 95% CI, 0.28-0.96; P = .04) but not at 5 years.
Discussion
Both structural and functional glaucoma progression outcomes were influenced by glaucoma genetic risk, with individuals in the top 5% of the PRS distribution progressing earlier than those in the bottom 95%, corresponding with a higher treatment intensity in the former. Since treatment is initiated or escalated in response to glaucoma worsening and is highly effective at slowing progression,11 we would expect the true association of PRS with glaucoma progression to be even greater and would also anticipate a similar association between PRS and time to treatment initiation or escalation. This raises the possibility that when combined with other clinical risk factors, a glaucoma PRS may be a valuable adjunct in determining treatment thresholds and intensity and the onset and frequency of glaucoma surveillance. Those in a high PRS group may deserve particular attention: despite more intensive treatment, these individuals remained more susceptible to progression and may therefore benefit from more intensive treatment including a lower-target IOP. Similarly, the lower-risk group may require less frequent monitoring or higher treatment threshold; however, our findings of the lower PRS group progressing slower did not persist at 5-year follow-up, which may be attributable to reduced statistical power or potentially a treatment effect (eg, more intensive treatment slowing down progression in other PRS groups).
Limitations
We were unable to directly investigate this due to the lack of untreated control arm of this cohort, with another limitation that treatment was at the discretion of the treating clinicians. Although an area of active investigation, a limitation of the glaucoma PRS used here was its derivation from European populations, which may not be transferable to other populations with different risk variant frequencies and effect sizes.
Conclusions
This work demonstrates an association between glaucoma polygenic risk and visual field and structural worsening in early glaucoma and supports further investigation in prospective clinical trials.
eAppendix
eReferences
References
- 1.Qassim A, Souzeau E, Hollitt G, Hassall MM, Siggs OM, Craig JE. Risk stratification and clinical utility of polygenic risk scores in ophthalmology. Transl Vis Sci Technol. 2021;10(6):14. doi: 10.1167/tvst.10.6.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig JE, Han X, Qassim A, et al. ; NEIGHBORHOOD consortium; UK Biobank Eye and Vision Consortium . Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet. 2020;52(2):160-166. doi: 10.1038/s41588-019-0556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan BJ, Bailey JC, Igo RP Jr, et al. Association of a primary open-angle glaucoma genetic risk score with earlier age at diagnosis. JAMA Ophthalmol. 2019;137(10):1190-1194. doi: 10.1001/jamaophthalmol.2019.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qassim A, Mullany S, Awadalla MS, et al. A polygenic risk score predicts intraocular pressure readings outside office hours and early morning spikes as measured by home tonometry. Ophthalmol Glaucoma. 2021;4(4):411-420. doi: 10.1016/j.ogla.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 5.Qassim A, Souzeau E, Siggs OM, et al. An intraocular pressure polygenic risk score stratifies multiple primary open-angle glaucoma parameters including treatment intensity. Ophthalmology. 2020;127(7):901-907. doi: 10.1016/j.ophtha.2019.12.025 [DOI] [PubMed] [Google Scholar]
- 6.Siggs OM, Han X, Qassim A, et al. Association of monogenic and polygenic risk with the prevalence of open-angle glaucoma. JAMA Ophthalmol. 2021;139(9):1023-1028. doi: 10.1001/jamaophthalmol.2021.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu M, Lin C, Weinreb RN, Lai G, Chiu V, Leung CKS. Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: a 5-year prospective study. Ophthalmology. 2016;123(6):1201-1210. doi: 10.1016/j.ophtha.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 8.Olsen CM, Green AC, Neale RE, et al. Cohort profile: the QSkin Sun and Health Study. Int J Epidemiol. 2012;41(4):929-929i. doi: 10.1093/ije/dys107 [DOI] [PubMed] [Google Scholar]
- 9.Seth NG, Kaushik S, Kaur S, Raj S, Pandav SS. 5-Year disease progression of patients across the glaucoma spectrum assessed by structural and functional tools. Br J Ophthalmol. 2018;102(6):802-807. doi: 10.1136/bjophthalmol-2017-310731 [DOI] [PubMed] [Google Scholar]
- 10.Chauhan BC, Vianna JR, Sharpe GP, et al. Differential effects of aging in the macular retinal layers, neuroretinal rim, and peripapillary retinal nerve fiber layer. Ophthalmology. 2020;127(2):177-185. doi: 10.1016/j.ophtha.2019.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295-1304. doi: 10.1016/S0140-6736(14)62111-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix
eReferences