Abstract
Viral infectious diseases remain a global public health problem. The rapid and widespread spread of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV‑2) has had a severe impact on the global economy and human activities, highlighting the vulnerability of humans to viral infectious diseases and the urgent need to develop new technologies and effective treatments. Organ-on-a-chip is an emerging technology for constructing the physiological and pathological microenvironment of human organs in vitro and has the advantages of portability, high throughput, low cost, and accurate simulation of the in vivo microenvironment. Indeed, organ-on-a-chip provides a low-cost alternative for investigating human organ physiology, organ diseases, toxicology, and drug efficacy. The lung is a main target organ of viral infection, and lung pathophysiology must be assessed after viral infection and treatment with antiviral drugs. This review introduces the construction of lung-on-a-chip and its related pathophysiological models, focusing on the in vitro simulation of viral infection and evaluation of antiviral drugs, providing a developmental direction for research and treatment of viral diseases.
Keywords: Lung-on-a-chip, Drug evaluation, Virus infection, SARS-CoV‑2
Graphical abstract
1. Introduction
Viral diseases have high morbidity and mortality rates worldwide (Schweitzer et al., 2015). Viruses that infect the lungs mainly include respiratory syncytial virus, influenza virus, rhinovirus, adenovirus, metapneumoviruses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 emerged at the end of 2019, has infected millions of people and killed more than one million people, and continues to spread worldwide (Li et al., 2020). Only a few treatments are available worldwide, and the efficacy of vaccines against SARS-CoV-2 variants remains to be determined (Kowalewski and Ray, 2020; McDonald and Holtz, 2020; Tatara, 2020; La Monica et al., 2022; Razonable et al., 2022).
In general, research and development of drugs and vaccines requires considerable time and money. In the past, research and development of drugs mainly relied on two-dimensional (2D) cell culture, three-dimensional (3D) system culture-based organoids and animal experiments. 2D cell culture in vitro is a common method for studying tissue pathophysiology and drug response, but it is difficult to reproduce the three-dimensional structure and biophysical and functional properties of the cellular microenvironment, and it does not mimic the inherent complex properties of tissues and organs (Hiemstra et al.,2019). Organoids based on three-dimensional (3D) culture systems are organ specific, multicellular, three-dimensional cultures that reproduce some of the key structural and functional properties of the corresponding organ. However, embryonic stem cells or induced pluripotent stem cells involve a long differentiation time, high cost and complicated operation (Sato et al., 2009). Animals are also used to mimic human pathophysiological microenvironments for disease research, preclinical drug development and screening. Nevertheless, due to species differences, substantial differences in physiological structure, tissue and organ functions, life support, and other parameters exist between animals and humans. Moreover, most pathogens are species specific, and animal models do not accurately simulate the physiological and pathological environment of the human body (Hartung, 2009; Snoeck, 2015). In addition, animal experimental research has limitations, such as a long model establishment cycle, high cost, low efficiency, a single research time point, and ethical issues (Perelson and Ribeiro, 2018). Therefore, animal models do not provide a strong rationale for the development and analysis of human lung models for disease modelling, drug development and screening in vitro. The inaccuracy of traditional models of simulating diseases and evaluating drugs is very likely to lead to the failure of clinical trials of candidate drugs and even serious drug toxicity, which damages the health of patients and increases the economic burden. In recent years, with the continuous advances in microfabrication and tissue engineering technologies, the construction of microfluidic organs-on-a-chip has enabled the building of biomimetic three-dimensional human tissue models, and the experimental results are more reliable than those obtained from 2D cell culture, 3D organoids or animals (Shahabipour et al., 2022; Tan et al., 2022).
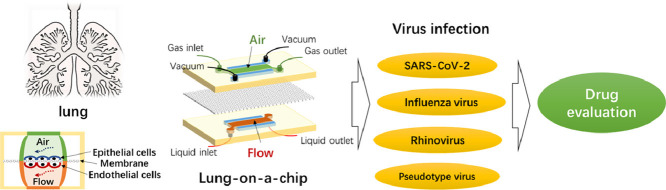
An organ-on-a-chip is a bionic device that uses microfluidic chips to simulate the main functions of human organs. It has micron-scale gas or fluid channels that simulate the tissue microenvironment and blood circulatory system and generate tissue-tissue and organ-organ interfaces, simulating the microenvironment, complex structure and biophysical factors of human organs. Indeed, an organ-on-a-chip can simulate organ physiological and pathological conditions more accurately than conventional cell culture in vitro. Furthermore, organs-on-a-chip may solve the shortcomings of animal experiments, such as long cycles, high costs and ethical issues. Overall, biomimetic organs-on-a-chip are expected to provide a low-cost alternative for studying human organ physiology, organ diseases, toxicology, and drug evaluation (Ingber, 2016, 2022; Li et al., 2019). As one of the earliest proposed and developed organs-on-a-chip, the lung-on-a-chip can be applied not only for model establishment and drug evaluation for diseases such as pulmonary oedema, pulmonary thrombosis, and lung tumours (Hassell et al., 2017; Huh et al., 2012; Jain et al., 2018; Yang et al., 2018) but also for lung diseases caused by viral infection (Deinhardt-Emmer et al., 2020; Nawroth et al., 2020). At present, the application of lung-on-a-chip in viral infection research is limited to a few studies, but with the continuation of the coronavirus disease 2019 (COVID-19) pandemic, the number of these studies will increase in the future.
In this article, we review the lung-on-a-chip model used to study viral infection and its application in the investigation of underlying disease mechanisms and drug evaluation. Challenges and related solutions for treating COVID-19 are presented. Finally, we discuss the modelling and drug evaluation using other organs-on-a-chip for COVID-19 and recommend new developmental directions for future studies in the fields of virology and drug evaluation using organ-on-a-chip technology, multiorgan-on-a-chip and body-on-a-chip platforms.
2. Construction of the lung-on-a-chip
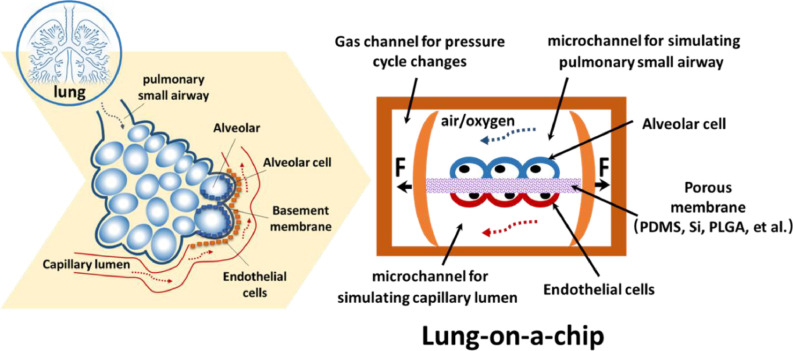
The main principle of the lung-on-a-chip is to use microfabrication to process microstructures of specific shapes on microchips composed of polydimethylsiloxane (PDMS), silicon or other high polymer materials. Two upper and lower microfluidic channel structures are used. The upper channel is seeded with alveolar epithelial cells and provides oxygen to form an air-liquid interface, simulating the alveolar structure; the lower channel contains vascular endothelial cells and is continuously perfused with culture medium, simulating capillary channels and fluid shears. The middle section is separated by a porous membrane to simulate the alveolar septum. Side chambers are located on both sides of the lung-on-a-chip that are connected to two vacuum pumps. By regularly changing the pressure of the side chambers, the porous membrane is regularly stretched to simulate breathing movement. After culture, the cells form a functional tissue unit called the lung-on-a-chip (Fig. 1 ). Airway-on-a-chip is another type of lung-on-a-chip. The construction of the airway-on-a-chip is similar to that of the alveolus-on-a-chip. The upper and lower microfluidic channels of the chip are composed of high polymer materials, the lower channel is seeded with vascular endothelial cells and continuously perfused with culture medium to simulate capillary channels and fluid shear stress, and the middle is separated by a porous membrane to simulate the airway septum. The difference is that the upper channel is seeded with primary human lung bronchus-airway epithelial basal stem cells, which can be induced to differentiate into ciliated cells, basal cells, club cells and goblet cells, thus accurately simulating airway function. The lung-on-a-chip material is the basis for its processing, manufacturing and application. Commonly used materials include PDMS, silicon, polylactic-co-glycolic acid (PLGA), and extracellular matrix (ECM), among others (Li et al., 2020; Rahimi et al., 2016; Xu et al., 2016; Zamprogno et al., 2021), with PDMS representing the most widely used material. Current technologies for manufacturing the lung-on-a-chip include classical lithography, moulding, microcontact imprinting, and 3D printing.
Fig. 1.
Schematic diagram of the alveolar structure. The microfabricated lung-on-a-chip uses compartmentalized PDMS microchannels to form an alveolar-capillary barrier on a thin, porous, flexible PDMS, silicon or PLGA membrane. Alveolar epithelial cells are seeded in the upper channel, which provide oxygen to form an air-liquid interface, simulating the alveolar structure. Vascular endothelial cells are seeded in the lower channel and are continuously perfused with culture medium, simulating capillary channels and fluid shear. The device recreates physiological breathing movements by applying a vacuum to the side chambers and causing mechanical stretching of the porous membrane.
In 2010, Huh D et al. designed a PDMS lung-on-a-chip model for the first time using soft lithography microfabrication technology and reproduced the respiratory function of alveoli in vitro (Huh et al., 2010). With the rapid development of microfabrication and tissue engineering technologies, the constructed lung-on-a-chip closely resembles the alveolar microenvironment of the human body. The lung-on-a-chip has been widely used to establish models of and evaluate drugs for pulmonary oedema, pulmonary thrombosis, lung tumours and other diseases. Huh D et al. constructed a lung-on-a-chip model to simulate pulmonary oedema. The results of the study showed that mechanical strain related to respiratory movement and IL-2 damage the function of the lung barrier, leading to pulmonary oedema. At the same time, the study identified potentially new therapies and found that angiopoietin-1 (Ang-1) and a new transient receptor potential vanilloid 4 (TRPV4) ion channel inhibitor (GSK2193874) may prevent IL-2 from exerting life-threatening toxicity (Huh et al., 2012). Jain A et al. used a lung-on-a-chip to simulate the formation of pulmonary thrombi, reproducing the finding that the endotoxin lipopolysaccharide (LPS) indirectly stimulates intravascular thrombus formation by activating the alveolar epithelium rather than by directly acting on endothelial cells. The findings reveal the cytoprotective and antithrombotic effects of a novel PAR-1 antagonist on acute lung injury and whole-blood perfusion, providing a new method to study the pathophysiological mechanism of human pulmonary thrombosis and promote drug development (Jain et al., 2018).
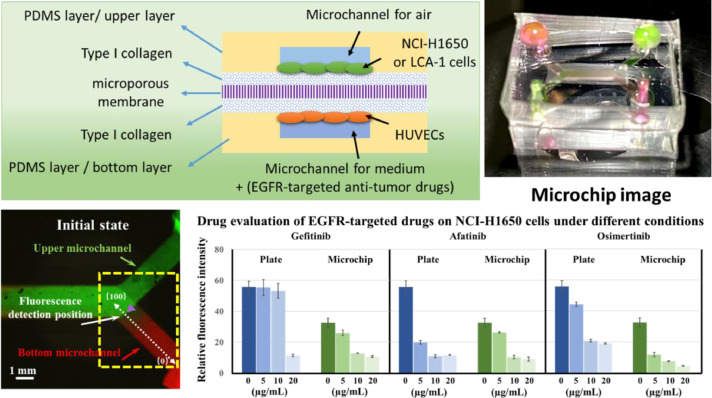
In addition to the abovementioned lung disease-related models, the lung-on-a-chip has been used to simulate lung tumour models and evaluate drugs, especially antitumour drugs. Yang X et al. fabricated a lung-on-a-chip using PLGA electrospun nanofibre membranes as chip substrates and cell scaffolds. The chip achieved 3D coculture of a human non-small cell lung cancer cell line (A549), human lung fibroblasts and human umbilical vein endothelial cells. The model was used to evaluate the efficacy of an epidermal growth factor receptor (EGFR)-targeting antitumour drug (gefitinib) (Yang et al., 2018). In our previous study, we also used 3D printing technology to prepare microfluidic chip templates and successfully constructed a PDMS-capsulated lung-on-a-chip based on resin membranes. The microporous membrane lung-on-a-chip simulated the lung tissue microenvironment, with coculture of lung cancer cells and vascular endothelial cells at different levels. We used two strategies, 2D cell culture and 3D lung-on-a-chip, to evaluate the efficacy of different EGFR-targeting drugs (gefitinib, afatinib and osimertinib) against NCI-H650 cells and primary lung cancer cells. The 3D lung-on-a-chip was better than 2D cell culture for evaluating drugs using the lung non-small cell lung cancer cell line NCI-H650 because it better matched existing clinical drug data. Because the 3D lung-on-a-chip may provide conditions that are more consistent with the physiological cell microenvironment, it has many advantages for examining primary tumour cells (Fig. 2 ) (Tan et al., 2022). In general, the lung-on-a-chip has shown great application potential in simulating lung disease models and evaluating drugs. The lung-on-a-chip can also be used to simulate viral infectious diseases and evaluate drugs.
Fig. 2.
Schematic diagram of the experimental principle and an image of the lung-on-a-chip. Fluorescence images and plots of fluorescence intensity profiles for characterization of small molecule diffusion in a microchip. Green, RH-123 aqueous solution. Red, RH-B aqueous solution. Scale bar = 1 mm. Effects of different concentrations of gefitinib, afatinib, and osimertinib on HCI-H1650 cells grown in the wells of a plate in 2D and in the 3D lung-on-a-chip. n = 3. Source: (Tan et al., 2022).
3. Application of the lung-on-a-chip for assessing viral infection
Viral infection is a leading cause of lung disease-related death. Viral infection can induce asthma exacerbation, seasonal flu and outbreaks of COVID-19. Asthma is considered a common chronic disease, and is present in 14% of all children worldwide and affects approximately 334 million people worldwide (Shrestha et al., 2020). Bronchopneumonia associated with seasonal influenza virus is one of the infectious diseases with the highest mortality rate. COVID-19, a new lung disease related to viral infection, has high infectivity and mortality, with millions of people infected and more than one million deaths; furthermore, many surviving patients have serious complications, causing serious damage to the global economy and human health and safety (Atkins et al., 2020; Nicola et al., 2020; Zhao et al., 2020). Because the virus is constantly mutating, the efficacy of existing drugs and vaccines against its variants is unclear. Therefore, the development of new research methods is urgently needed to simulate the physiological and pathological changes associated with pulmonary diseases after viral infection and to develop and evaluate antiviral drugs. We summarize the latest research on the use of the lung-on-a-chip for simulating viral infection and evaluating drugs, as shown in Table 1 .
Table 1.
List for various studies of viral infection using the lung-on-a-chip.
| Organ-on-a-chip | Virus | Cell types | Design features | Factors investigated | Drug evaluation | Ref. |
|---|---|---|---|---|---|---|
| Alveolus-on-a-chip | SARS-CoV-2 | Alveolar epithelial cells (type II) | Two channels separated by a microporous PDMS membrane | Recapitulation of lung injury and immune responses induced by SARS-CoV-2 at the organ level | Remdesivir inhibits viral replication and alleviates barrier disruption | (Zhang et al., 2021) |
| Lung microvascular cells (HULEC-5a) | Upper alveolar epithelial channel | Antiviral drug efficacy | ||||
| Human peripheral blood mononuclear cells | Lower microvascular endothelial channel under fluid flow | |||||
| Alveolus-on-a-chip | Pseudotyped SARS-CoV-2Poly(I:C) | Alveolar epithelial cells (type II) | A collagen gel channel in the centre and two shouldered cell culture channels | Recapitulation of key physiological characteristics of human alveolar units | A monoclonal antibody effectively inhibits the entry of viruses into host cells | (Cao et al., 2022) |
| Primary human umbilical vein endothelial cells (HUVECs) | Simulation of air mechanical strain and blood shear stress | Exploration of virus pathogenesis induced by a pseudovirus and poly(I:C) | ||||
| U937 cells | Monoclonal antibody | |||||
| Alveolus-on-a-chip | SARS-CoV-2 | Primary human alveolar epithelial cells (type I and type II) | Two channels separated by a PDMS membrane | Comparation of the differences between alveolar epithelial cells and vascular endothelial cells induced by SARS-CoV-2 at the organ level | Tocilizumab does not reduce all of the endothelial cell damage | (Thacker et al., 2021) |
| Human lung microvascular endothelial cells | Upper alveolar epithelial channel with an air-liquid interface | Anti-IL-6R monoclonal antibody | ||||
| Lower microvascular endothelial channel under fluid flow | ||||||
| Airway-on-a-chip | Influenza A | Primary human lung bronchial-airway epithelial basal stem cells | Two microchannels separated by an | Simulation of viral infection, strain-dependent virulence, cytokine production and the recruitment of circulating immune cells | Coadministration of nafamostat with oseltamivir doubled the treatment time window for oseltamivir | (Si et al., 2021b) |
| Pseudotyped SARS-CoV-2 | Primary human pulmonary microvascular endothelial cells | ECM-coated porous membrane | Antiviral drugs targeting influenza and SARS-CoV-2 | Amodiaquine inhibited SARS-CoV-2 infection of cells in chips and in hamsters | ||
| Neutrophils | Upper airway channel with an air-liquid interface | |||||
| Lower vascular channel under fluid flow | ||||||
| Alveolus-on-a-chip | Influenza virus | Alveolar epithelial cells (NCI-H441) | Two microchannels separated by a PET membrane | Establishing an alveolus-on-a-chip composed of vascular and epithelial cell structures with macrophages | None | (Deinhardt-Emmer et al., 2020) |
| HUVECs | Upper channel with alveolar epithelial cells and macrophages | The effects of flow conditions and macrophages on barrier function | ||||
| Macrophages | Lower channel with endothelial cells and medium flow generated by a pump system | The effects of viral infection or coinfection | ||||
| Airway chip | Influenza A | Primary human lung airway epithelial cells (HLAECs) | Two parallel microchannels separated by an ECM-coated membrane | Simulation of pathophysiology and mutation during influenza virus infection | Amantadine, nafamostat and oseltamivir prevent influenza virus | (Si et al., 2021a) |
| Human pulmonary microvascular endothelial cells (HPMVECs) | Upper airway channel with an air-liquid interface | Modelling human-to-human transmission of infection with continued antiviral drug treatment | The combination of nafamostat with oseltamivir extends the therapeutic window from 48 h to 96 h | |||
| Lower vascular channel under medium flow | Assessing antiviral drugs and antiviral resistance | |||||
| Airway lung-chip | Human rhinovirus 16 (HRV16) | Human primary airway epithelial cells (hAECs) | Two microchannels separated by a pore polyester membrane | First recapitulation of HRV infection of the asthmatic epithelium and complex features of virus-induced asthma exacerbation | A CXCR2 antagonist (MK-7123) reduced adhesion, motility, and transmigration of neutrophils | (Nawroth et al., 2020) |
| HUVECs | Upper mucociliary airway epithelium with an air-liquid interface | The effects of IL-13 on rhinovirus infection | IL-13 may impair the hosts’ ability | |||
| Neutrophils | Lower microvascular endothelium under fluid flow | Antiviral drugs | ||||
| Airway-on-a-chip | Poly(I:C) | Human primary airway epithelial cells (hAECs) | Two microchannels separated by a semiporous poly-ester membrane | Analysis of organ-level lung pathophysiology of human lung inflammation in vitro | Dexamethasone, tofacitinib, budesonide and a BRD4 inhibitor inhibit cytokine-induced recruitment of circulating neutrophils | (Benam et al., 2016) |
| Human pulmonary microvascular endothelial cells | Upper airway channel with an air-liquid interface | Anti-inflammatory compounds | ||||
| HUVECs | Lower vascular channel under medium flow | |||||
| Gut-on-a-chip | SARS-CoV-2 | Caco-2 cells | Two channels separated by a porous PDMS membrane | Creating an intestinal infection on a chip | None | (Guo et al., 2021) |
| HT-29 cells | Upper intestinal epithelial channel | Recapitulation of the key features of changes in the intestinal epithelium-vascular endothelium barrier induced by SARSCoV-2 | ||||
| HUVECs | Lower vascular endothelial channel under fluid flow | |||||
| Human peripheral blood mononuclear cells (PBMCs) | ||||||
| Vasculature-on-a-c-hip | HCoV-NL63 virus | HUVECs | A scaffold with a square luminal structure | Simulation of the interaction of virus alone with the endothelialized vasculature-on-a-chip | (Lu et al., 2022) | |
| SARS-CoV-2 | Human PBMCs | The bioscaffold was placed on patterned hot-embossed polystyrene base plate and it is bonded onto the 96-well bottomless plate | Studies of the interaction of the SARS-CoV-2 and (HCoV)-NL63 exposed-endothelial cells with PBMCs | QHREDGS significantly attenuates the inflammatory state of cells infected with SARS-CoV-2 | ||
| Antiviral drugs |
3.1. Lung-on-a-chip for rhinovirus-related asthma
Viral infections, particularly rhinovirus (RV), are a major cause of asthma exacerbation. Antiviral cytokines produced from alveolar epithelial cells or dendritic cells are present at lower levels in patients with asthma or those with high IgE levels, which may contribute to virus-induced aggravation of the disease (Nakagome and Nagata, 2022). Nawroth J. C. et al. used a human airway lung-on-a-chip model to show for the first time that live human rhinovirus (HRV) infects asthmatic epithelial cells, reproducing the complex features of virus-induced asthma exacerbations. The dynamic microenvironment of the chip enables a real-time study of viral infection, epithelial cell responses and immune cell recruitment under healthy and asthmatic conditions, reproducing key features of patients with asthma and rhinovirus-infected individuals, including ciliary cell shedding, altered ciliary sweep frequency, cupping proliferation of microvascular endothelial cells, increased expression of adhesion molecules in microvascular endothelial cells and release of inflammatory mediators. The results suggest that a CXCR2 antagonist (MK-7123) reduces neutrophil adhesion, motility and transport; IL-13 may impair the ability of the host to mount an appropriate and coordinated immune response to RV (Nawroth et al., 2020). The study was the first to simulate and validate key features of virus-induced asthma exacerbation using a lung-on-a-chip. Unfortunately, the effect of breathing exercise on asthma exacerbation has not been investigated to date.
3.2. Lung-on-a-chip for influenza virus infection
Seasonal influenza virus-associated bronchopneumonia has a high mortality rate. The greatest challenge to fight against influenza virus infection is the rapid evolution of viruses, which leads to the emergence of variant strains and renders existing anti-influenza drugs and vaccines ineffective (Du X et al., 2017). Therefore, developing more effective methods to control influenza will require better prediction of the evolution of virus resistance to treatments, as well as more rapid development of new drugs and vaccines, both of which are currently limited by the lack of relevant preclinical models (Krammer et al., 2018).
Benam K. H et al. constructed an airway-on-a-chip and then used it to simulate the pathophysiological changes associated with asthma and lung inflammation. The virus analogue poly(I:C) or lipopolysaccharide (LPS) was used to simulate asthma exacerbation and the therapeutic effect of drugs was evaluated using an airway-on-a-chip. The characteristics of chronic obstructive pulmonary disease were reproduced by an airway-on-a-chip lined with epithelial cells from individuals with chronic obstructive pulmonary disease. Exposure of epithelial cells to interleukin-13 (IL-13) reconstructed goblet cell proliferation, excessive cytokine secretion and decreased ciliary function in patients with asthma, and dexamethasone, tofacitinib, budesonide, and BRD4 inhibitors inhibited cytokine-induced recruitment of circulating neutrophils (Benam et al., 2016). Using this airway-on-a-chip for simulating inflammatory diseases in the human lung, researchers have assessed the synergistic effect of the lung endothelium and epithelium on cytokine secretion and identified new biomarkers of disease progression. In another study, Si et al. employed a human airway-on-a-chip to establish an in vitro model of human influenza infection related to physiology and clinical practice, which may be used to predict potential emerging viruses. Modelling human-to-human transmission of infection in the presence of persistent antiviral drugs using the lung-on-a-chip enabled researchers to determine the emergence of 20 clinically prevalent mutations that lead to resistance to amantadine and oseltamivir, along with the discovery of new drug resistance mutations that may cause an influenza pandemic (Si et al., 2021a). The model has also been used for a preclinical evaluation of new anti-influenza treatments to determine candidate anti-influenza drugs. In addition, Deinhardt-Emmer S et al. used an alveolus-on-a-chip model in vitro to study the effect of influenza virus and Staphylococcus aureus coinfection on endothelial cells. Monitoring the spatiotemporal transmission, characteristic morphology and functional changes of pathogens on the alveolus-on-a-chip showed that the flow conditions and the presence of macrophages increase the barrier function, and the high barrier integrity was maintained for 14 days. The findings showed that infection of epithelial cells caused a strong inflammatory response that spread to the endothelial cells. Although the integrity of the epithelium was not compromised by a single viral infection or coinfection, endothelial cell damage correlated with the loss of barrier function (Deinhardt-Emmer et al., 2020). The biomimetic alveolus-on-a-chip used in the study provides a promising platform for exploring the mechanism of host‒pathogen interactions and identifying molecular and cellular targets for new treatment strategies for pneumonia, but no research or evaluation of related antiviral drugs has been conducted. Moreover,breathing motions can suppress viral replication by activating protective innate immune responses in epithelial and endothelial cells in human lung alveolus chip, which are mediated in part through activation of the mechanosensitive ion channel TRPV4 and signaling via receptor for advanced glycation end products (RAGE) (Bai et al., 2022).
In general, with the progress of lung-on-a-chip technology and the in-depth study of viral infection, researchers have not only established lung-on-a-chip models for viral infection and drug evaluations but also a model of human-to-human infection transmission to discover new drug resistance-related mutations and determine candidate anti-influenza drugs. Lung-on-a-chip models of various microbial infections have even been established.
3.3. Lung-on-a-chip for SARS-CoV-2 infection
COVID-19 is a severe pneumonia pandemic caused by SARS-CoV-2 that emerged at the end of 2019 and then quickly spread around the world (Li et al., 2020; Wang et al., 2021). However, the lack of suitable preclinical models for assessing the pathogenesis of SARS-CoV-2 and the rapid emergence of multiple SARS-CoV-2 variants has accelerated the spread of the virus, which has also led to continued failure of drug treatments and vaccine designs (Kowalewski and Ray, 2020; McDonald and Holtz, 2020; Tatara, 2020). Thacker VV et al. utilized a vascularized lung-on-a-chip model to study the effects of SARS-CoV-2 infection on the vascular endothelium. Infection of alveolar epithelial cells resulted in limited apical release of virions, consistent with reports of monoculture infection. Viral RNA persists in a single cell that produces an inflammatory response, which is transient in epithelial cells but persists in endothelial cells, and these cells secrete IL-6 even in the absence of immune cells. Loss of barrier integrity was reduced but not completely prevented with an inhibitor of IL-6 signalling (tocilizumab) (Thacker et al., 2021). Thus, SARS-CoV-2-mediated endothelial cell injury is not directly related to cytokine storms. Additionally, Zhang M et al. constructed a biomimetic human disease-on-a-chip model that was able to reproduce the lung injury and immune response induced by SARS-CoV-2 at the organ level in vitro. Coculture of human alveolar epithelial cells, microvascular endothelial cells, and circulating immune cells in a fluidic state reproduced the main features of the normal human alveolar-capillary barrier. Epithelial cells are more sensitive to the virus than endothelial cells during SARS-CoV-2 infection, and a transcriptional analysis showed that the innate immune response and cytokine-dependent pathway of endothelial cells were activated at 3 days after infection, revealing distinct responses across cell types. Viral infection results in increased immune cell recruitment, endothelial shedding, and inflammatory cytokine release, suggesting that immune cells play a critical role in alveolar barrier damage and increased inflammation. Remdesivir inhibits viral replication and mitigates chip barrier disruption (Zhang et al., 2021). The lung-on-a-chip model may closely reflect the human response to SARS-CoV-2 infection, which is difficult to achieve with in vitro models and provides a unique platform for COVID-19 research and drug development. Nevertheless, this study has some shortcomings: one cell type of alveolar epithelium does not completely simulate the primary alveolar tissue, and only one candidate antiviral drug was evaluated.
The two studies described above mainly used the airway-on-a-chip to simulate SARS-CoV-2 infection, revealing the physiological and pathological changes in the alveolar epithelium and vascular endothelium after viral infection, but few studies have focused on improving the lung-on-a-chip or developing and evaluating drugs. Si L et al. constructed a microfluidic bronchial airway-on-a-chip using lung bronchial-airway epithelial basal stem cells and lung endothelial cells that simulates viral infection, strain-dependent virulence, cytokine production, and recruitment of circulating immune cells. The combination of nafamostat and oseltamivir doubled the treatment time window for oseltamivir in influenza A-infected airways-on-a-chip. For the chip infected with SARS-CoV-2 pseudovirus, clinically relevant doses of the antimalarial drug amodiaquine inhibited infection, but clinical doses of hydroxychloroquine and other antiviral drugs that inhibit the entry of pseudotyped SARS-CoV-2 in cell lines under static conditions did not.Amodiaquine reportedly exerts significant preventive and therapeutic effects on hamsters infected with SARS-CoV-2 (Si et al., 2021b) The lung-on-a-chip used in that study both simulates SARS-CoV-2 infection and evaluates drugs and has been used for influenza virus infection and drug evaluation, realizing a chip that simulates multiple viral infections. Cao T et al. used a type I collagen gel to simulate the respiratory membrane, and circulating air vibration was applied to simulate respiratory movement, along with the construction of a three-dimensional alveolus-on-a-chip with mechanical strain and an extracellular matrix. The alveolus-on-a-chip reproduces key physiological features of human alveolar units, which lays the foundation for viral infection studies at the organ level. Virus pathogenesis and the blocking ability of antibodies during viral infection have been explored using virus analogue poly(I:C) and SARS-CoV-2 pseudovirus, and the results showed that monoclonal antibodies effectively inhibit virus entry into host cells (Cao et al., 2022). The innovation of this research is that the type I collagen gel simulates the respiratory membrane, the circulating air vibration simulates respiratory movement, and the constructed alveolus-on-a-chip closely resembles the physiological microenvironment of alveoli. This alveolus-on-a-chip provides a good platform for SARS-CoV-2-related research and has great potential for organ-level studies of human lung physiology and pathophysiology in vitro. However, the study only evaluated monoclonal antibodies.
4. Other organs-on-a-chip for assessing SARS-CoV-2 infection
SARS-CoV-2 infection is characterized by asymptomatic and mild disease to severe systemic symptoms, involving multiple organs, such as the lungs (McGonagle et al., 2020), blood vessels (Siddiqi et al., 2021), gastrointestinal tract (Lin et al., 2020) and heart (Chen et al., 2020), and ultimately results in organ failure (Wiersinga et al., 2020). As our understanding of disease pathology improves, accumulating evidence indicates that vascular and gastrointestinal pathology may play an important role in COVID-19 outcomes (Becker, 2020; Qian et al., 2021; Xiao et al., 2020). This clinical evidence suggests that the gastrointestinal tract and blood vessels are two other high-risk organs for SARS-CoV-2, but the physiological and pathological mechanisms involving the gastrointestinal tract and blood vessels after SARS-CoV-2 infection are unclear, and related preclinical models to evaluate antiviral drugs are lacking. Table 1 describes the latest research on the application of vasculature-on-a-chip and gut-on-a-chip to simulate SARS-CoV-2 infection and evaluate drugs.
Guo Y et al. constructed an intestinal infection-on-a-chip model to reproduce the human intestinal pathophysiology induced by SARS-CoV-2 at the organ level. This microengineered gut-on-a-chip reconstitutes key features of the intestinal epithelial-vascular endothelial barrier, enabling 3D coculture of human intestinal epithelial cells, mucus-secreting cells, and vascular endothelial cells under physiological fluid flow. The intestinal epithelial cells were susceptible to viral infection, with obvious morphological changes, intestinal villus damage, scattered mucus-secreting cells and decreased E-cadherin expression, suggesting that the virus destroys the integrity of the intestinal barrier. A transcriptional analysis revealed abnormal metabolism of RNA and proteins in epithelial cells and endothelial cells after viral infection, as well as an activated immune response, which may lead to intestinal barrier injury related to gastrointestinal symptoms (Guo et al., 2021). This human organ system may partially reflect intestinal barrier damage and the human response to viral infection, which is not possible in other existing culture models in vitro, providing a unique and rapid platform to accelerate COVID-19 research and develop new therapies. Regardless, the study did not develop and evaluate antiviral drugs. In addition, Lu R et al. developed a vasculature-on-a-chip to study SARS-CoV-2 infection, first simulating the interaction of the virus alone with the endothelialized vasculature on the chip and then studying endothelial cells exposed to the virus and peripheral blood mononuclear cells (PBMCs). Human coronavirus (HCoV)-NL63 and SARS-CoV-2 reduce endothelial barrier function by disrupting VE-cadherin junctions and increasing the levels of proinflammatory cytokines. Compared with HCoV-NL63 infection, inflammatory cytokine levels are significantly increased in SARS-CoV-2 infection. After SARS-CoV-2 infection, PBMCs were introduced into the vasculature-on-a-chip, which further aggravated endothelial dysfunction induced by cytokines, indicating that the intercellular crosstalk between endothelial cells and monocytes jointly promotes the high inflammatory state. An angiopoietin-1-derived peptide (QHREDGS) has also been identified as a potential therapeutic agent that substantially attenuates the inflammatory state of cells, thereby improving barrier function and the survival of endothelial cells following SARS-CoV-2 infection in the presence of PBMCs (Lu et al., 2022). The vasculature-on-a-chip in the study not only simulated the vascular model after viral infection but also facilitated an exploration of the interaction between endothelial cells and monocytes and comparing changes in the vascular endothelium after infection with different types of virus. Finally, the therapeutic effect of antiviral drugs was evaluated.
5. Conclusions and prospects
Despite some progress in preventing viral infectious diseases, viruses still pose a serious threat to human health. In particular, with the emergence of the COVID-19 pandemic, new variants of SARS-CoV-2, such as Alpha, Beta, Gamma, Mu, Delta and Omicron, pose new challenges to existing antiviral drugs (such as paxlovid) and vaccines (Callaway, 2022; Chavda et al., 2022; Service, 2022). Therefore, various approaches must be mobilized to study human viral infections and to develop appropriate drugs and vaccines. Starting from the construction of the lung-on-a-chip by introducing the establishment of the model and evaluation of drugs for pulmonary oedema, pulmonary thrombosis, lung tumour and other diseases using the lung-on-a-chip, this article focuses on the simulation of viral infection in vitro and the evaluation of antiviral drugs using the lung-on-a-chip. The lung is not the only organ infected with the virus; indeed, SARS-CoV-2 infection also targets multiple organs, such as the blood vessels, intestines, heart and kidneys. Hence, a single lung-on-a-chip does not reproduce the whole process of viral infection in the human body. The construction of organs-on-a-chip and their application in viral infection often involve the cooperation of multiple disciplines, such as materials science, tissue engineering and virology, which limits the development of research to a certain extent. In addition, the current research on SARS-CoV-2 must be conducted in laboratories with biosafety levels of 3 and above, and these laboratory requirements have significantly increased the difficulty of research on COVID-19.
Although the application of organs-on-chip to viral infection and drug evaluation faces many challenges, with the development of microfabrication, tissue engineering and in-depth research on viral infection, the combination of multiple organs-on-a-chip or body-on-a-chip is the direction of future development. Compared with the traditional methods of building models, the lung-on-a-chip has many advantages, such as portability, high throughput, low cost, and simulation of the in vivo microenvironment, but it also faces some challenges (Francis et al., 2022; Ingber, 2022; Low et al., 2021). PDMS is the most commonly used material for manufacturing a lung-on-a-chip, which has the problem of absorbing hydrophobic drugs, thus limiting its application in manufacturing organs-on-a-chip for drug discovery, development and application. However, researchers continue to try new manufacturing materials, which is expected to solve the problem. Moreover, at present, the thickness of the barrier membrane of the lung-on-a-chip is approximately 10 μm, but the actual thickness of the alveolar septum is less than 1 μm. New materials and new manufacturing technologies may produce ultrathin membranes similar to the thickness of alveolar septa in the future. At present, apart from technical problems, perhaps the biggest challenge is the need for sufficient credible data that demonstrates the advantages of human lung-on-a-chip over animal models before some academic research institutes and pharmaceutical companies accept this new technology into their laboratories (Ingber, 2022) Collaborative and interdisciplinary research is also expected to address current research bottlenecks (Sun et al., 2021; Tang et al., 2020; Tao et al., 2022). Poly(I:C) virus analogues and SARS-CoV-2 pseudovirus can be used as alternatives to viruses. In conclusion, an increasing number of studies have documented the use of lung-on-a-chip and organ-on-a-chip as reliable microphysiological tools to reproduce the microenvironment of viral infection and conduct drug evaluations in vivo, and they can be used as a complement or alternative to 2D cultures, 3D organoids and animal experiments.
Funding
This work was supported by Shenzhen Hospital of Southern Medical University, Research Promotion Funds for the Key Discipline Construction Program (No. ZCXM2022XZ000705).
Ethics approval
Not Applicable
Data availability
No data was used for the research described in the article.
CRediT authorship contribution statement
Jianfeng Tan: Conceptualization, Investigation, Writing – original draft, Funding acquisition. Quanwei Guo: Conceptualization, Investigation, Writing – original draft, Funding acquisition. Lingling Tian: Investigation, Writing – original draft. Zhendong Pei: Writing – original draft, Writing – review & editing. Dongfang Li: Investigation. Mengxi Wu: Investigation. Jianhua Zhang: Writing – original draft, Writing – review & editing, Funding acquisition. Xinghua Gao: Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Data Availability
No data was used for the research described in the article.
References
- Atkins J.L., Masoli J., Delgado J., Pilling L.C., Kuo C.L., Kuchel G.A., Melzer D. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H., Si L., Jiang A., Belgur C., Zhai Y., Plebani R., Oh C.Y., Rodas M., Patil A., Nurani A., Gilpin S.E., Powers R.K., Goyal G., Prantil-Baun R., Ingber D.E. Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip. Nat. Commun. 2022;13(1) doi: 10.1038/s41467-022-29562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R.C. Anticipating the long-term cardiovascular effects of COVID-19. J. Thromb. Thrombolysis. 2020;50(3):512–524. doi: 10.1007/s11239-020-02266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benam K.H., Villenave R., Lucchesi C., Varone A., Hubeau C., Lee H., Alves S.E., Salmon M., Ferrante T.C., Weaver J.C., Bahinski A., Hamilton G.A., Ingber D.E. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods. 2016;13(2):151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- Callaway E. COVID rebound is surprisingly common - even without Paxlovid. Nature. 2022 doi: 10.1038/d41586-022-02121-z. [DOI] [PubMed] [Google Scholar]
- Cao T., Shao C., Yu X., Xie R., Yang C., Sun Y., Yang S., He W., Xu Y., Fan Q., Ye F. Biomimetic Alveolus-on-a-Chip for SARS-CoV-2 infection recapitulation. Research (Wash D C) 2022;2022 doi: 10.34133/2022/9819154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V.P., Vuppu S., Mishra T., Kamaraj S., Patel A.B., Sharma N., Chen Z.S. Recent review of COVID-19 management: diagnosis, treatment and vaccination. Pharmacol. Rep. 2022 doi: 10.1007/s43440-022-00425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt-Emmer S., Rennert K., Schicke E., Cseresnyés Z., Windolph M., Nietzsche S., Heller R., Siwczak F., Haupt K.F., Carlstedt S., Schacke M., Figge M.T., Ehrhardt C., Löffler B, Mosig A.S. Co-infection with Staphylococcus aureus after primary influenza virus infection leads to damage of the endothelium in a human alveolus-on-a-chip model. Biofabrication. 2020;12(2):25012. doi: 10.1088/1758-5090/ab7073. [DOI] [PubMed] [Google Scholar]
- Du X, King A.A., Woods R.J., Pascual M. Evolution-informed forecasting of seasonal influenza a (H3N2) Sci. Transl. Med. 2017;9(413) doi: 10.1126/scitranslmed.aan5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis I., Shrestha J., Paudel K.R., Hansbro P.M., Warkiani M.E., Saha S.C. Recent advances in lung-on-a-chip models. Drug Discov. Today. 2022;27(9):2593–2602. doi: 10.1016/j.drudis.2022.06.004. [DOI] [PubMed] [Google Scholar]
- Guo Y., Luo R., Wang Y., Deng P., Song T., Zhang M., Wang P., Zhang X., Cui K., Tao T., Li Z., Chen W., Zheng Y, Qin J. SARS-CoV-2 induced intestinal responses with a biomimetic human gut-on-chip. Sci. Bull. (Beijing) 2021;66(8):783–793. doi: 10.1016/j.scib.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T. A toxicology for the 21st century–mapping the road ahead. Toxicol. Sci. 2009;109(1):18–23. doi: 10.1093/toxsci/kfp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell B.A., Goyal G., Lee E., Sontheimer-Phelps A., Levy O., Chen C.S., Ingber D.E. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017;21(2):508–516. doi: 10.1016/j.celrep.2017.09.043. [DOI] [PubMed] [Google Scholar]
- Hiemstra P.S., Tetley T.D., Janes S.M. Airway and alveolar epithelial cells in culture. Eur. Respir. J. 2019;54(5) doi: 10.1183/13993003.00742-2019. [DOI] [PubMed] [Google Scholar]
- Huh D., Leslie D.C., Matthews B.D., Fraser J.P., Jurek S., Hamilton G.A., Thorneloe K.S., Mcalexander M.A., Ingber D.E. A human disease model of drug toxicity–induced pulmonary edema in a Lung-on-a-Chip microdevice. Sci. Transl. Med. 2012;4(159) doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D., Matthews B.D., Mammoto A., Montoya-Zavala M., Hsin H.Y., Ingber D.E. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D.E. Reverse engineering human pathophysiology with Organs-on-Chips. Cell. 2016;164(6):1105–1109. doi: 10.1016/j.cell.2016.02.049. [DOI] [PubMed] [Google Scholar]
- Ingber D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022;23(8):467–491. doi: 10.1038/s41576-022-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Barrile R., van der Meer A.D., Mammoto A., Mammoto T., Ceunynck K., Aisiku O., Otieno M.A., Louden C.S., Hamilton G.A., Flaumenhaft R., Ingber D.E. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin. Pharmacol. Ther. 2018;103(2):332–340. doi: 10.1002/cpt.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewski J., Ray A. Predicting novel drugs for SARS-CoV-2 using machine learning from a >10 million chemical space. Heliyon. 2020;6(8):e4639. doi: 10.1016/j.heliyon.2020.e04639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F., Smith G., Fouchier R., Peiris M., Kedzierska K., Doherty P.C., Palese P., Shaw M.L., Treanor J., Webster R.G., García-Sastre A. Influenza. Nat. Rev. Dis. Primers. 2018;4(1):3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Monica G., Bono A., Lauria A., Martorana A. Targeting SARS-CoV-2 main protease for treatment of COVID-19: covalent inhibitors structure-activity relationship insights and evolution perspectives. J. Med. Chem. 2022 doi: 10.1021/acs.jmedchem.2c01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Yang X., Xue C., Zhao L., Zhang Y., Gao X. Biomimetic human lung-on-a-chip for modeling disease investigation. Biomicrofluidics. 2019;13(3):31501. doi: 10.1063/1.5100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Sun X., Ji B., Yang X., Zhou B., Lu Z., Gao X. PLGA Nanofiber/PDMS microporous composite Membrane-Sandwiched microchip for drug testing. Micromachines. 2020;11(12) doi: 10.3390/mi11121054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Gu Z., Gao L., Shi H., Mai L., Liu Y., Lin X., Lai R., Yan Z., Li X., Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- Low L.A., Mummery C., Berridge B.R., Austin C.P., Tagle D.A. Organs-on-chips: into the next decade. Nat. Rev. Drug Discov. 2021;20(5):345–361. doi: 10.1038/s41573-020-0079-3. [DOI] [PubMed] [Google Scholar]
- Lu R., Lai B., Rafatian N., Gustafson D., Campbell S.B., Banerjee A., Kozak R., Mossman K., Mubareka S., Howe K.L., Fish J.E., Radisic M. Vasculature-on-a-chip platform with innate immunity enables identification of angiopoietin-1 derived peptide as a therapeutic for SARS-CoV-2 induced inflammation. Lab Chip. 2022;22(6):1171–1186. doi: 10.1039/d1lc00817j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald K.A., Holtz R.B. From farm to finger Prick-A perspective on how plants can help in the fight against COVID-19. Front. Bioeng. Biotechnol. 2020;8:782. doi: 10.3389/fbioe.2020.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagome K., Nagata M. Innate immune responses by respiratory viruses, including rhinovirus, during asthma exacerbation. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.865973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth J.C., Lucchesi C., Cheng D., Shukla A., Ngyuen J., Shroff T., Varone A., Karalis K., Lee H., Alves S., Hamilton G.A., Salmon M., Villenave R. A microengineered airway lung chip models key features of viral-induced exacerbation of asthma. Am. J. Resp. Cell Mol. 2020;63(5):591–600. doi: 10.1165/rcmb.2020-0010MA. [DOI] [PubMed] [Google Scholar]
- Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson A.S., Ribeiro R.M. Introduction to modeling viral infections and immunity. Immunol. Rev. 2018;285(1):5–8. doi: 10.1111/imr.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q., Fan L., Liu W., Li J., Yue J., Wang M., Ke X., Yin Y., Chen Q., Jiang C. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin. Infect. Dis. 2021;73(3):361–366. doi: 10.1093/cid/ciaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R., Htwe S.S., Ochoa M., Donaldson A., Zieger M., Sood R., Tamayol A., Khademhosseini A., Ghaemmaghami A.M., Ziaie B. A paper-based in vitro model for on-chip investigation of the human respiratory system. Lab. Chip. 2016;16(22):4319–4325. doi: 10.1039/c6lc00866f. [DOI] [PubMed] [Google Scholar]
- Razonable R.R., O'Horo J.C., Hanson S.N., Arndt R.F., Speicher L.L., Seville T.A., Hall S.T., Pike M.L., Heyliger A., Larsen J.J., Ganesh R., Tulledge-Scheitel S.M. Outcomes of bebtelovimab treatment is comparable to ritonavir-boosted nirmatrelvir among high-risk patients with coronavirus disease-2019 during SARS-CoV-2 BA.2 omicron epoch. J. Infect. Dis. 2022 doi: 10.1093/infdis/jiac346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- Service R.F. Bad news for Paxlovid? Resistance may be coming. Science. 2022;377(6602):138–139. doi: 10.1126/science.add8037. [DOI] [PubMed] [Google Scholar]
- Shahabipour F., Satta S., Mahmoodi M., Sun A., de Barros N.R., Li S., Hsiai T.K., Ashammakhi N. Engineering organ-on-a-chip systems to model viral infections. Biofabrication. 2022 doi: 10.1088/1758-5090/ac6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha J., Razavi B.S., Aboulkheyr E.H., Yaghobian A.D., Thierry B., Ebrahimi W.M., Ghadiri M. Lung-on-a-chip: the future of respiratory disease models and pharmacological studies. Crit. Rev. Biotechnol. 2020;40(2):213–230. doi: 10.1080/07388551.2019.1710458. [DOI] [PubMed] [Google Scholar]
- Si L., Bai H., Oh C.Y., Jin L., Prantil-Baun R., Ingber D.E. Clinically relevant influenza virus evolution reconstituted in a human lung Airway-on-a-Chip. Microbiol. Spectr. 2021;9(2):e25721. doi: 10.1128/Spectrum.00257-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si L., Bai H., Rodas M., Cao W., Oh C.Y., Jiang A., Moller R., Hoagland D., Oishi K., Horiuchi S., Uhl S., Blanco-Melo D., Albrecht R.A., Liu W.C., Jordan T., Nilsson-Payant B.E., Golynker I., Frere J., Logue J., Haupt R., Mcgrath M., Weston S., Zhang T., Plebani R., Soong M., Nurani A., Kim S.M., Zhu D.Y., Benam K.H., Goyal G., Gilpin S.E., Prantil-Baun R., Gygi S.P., Powers R.K., Carlson K.E., Frieman M., Tenoever B.R., Ingber D.E. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat. Biomed. Eng. 2021;5(8):815–829. doi: 10.1038/s41551-021-00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Libby P, Ridker P.M. COVID-19 - a vascular disease. Trends Cardiovasc. Med. 2021;31(1):1–5. doi: 10.1016/j.tcm.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck H.W. Modeling human lung development and disease using pluripotent stem cells. Development. 2015;142(1):13–16. doi: 10.1242/dev.115469. [DOI] [PubMed] [Google Scholar]
- Sun A.M., Hoffman T., Luu B.Q., Ashammakhi N, Li S. Application of lung microphysiological systems to COVID-19 modeling and drug discovery: a review. Biodes. Manuf. 2021;4(4):1–19. doi: 10.1007/s42242-021-00136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Sun X., Zhang J., Li H., Kuang J., Xu L., Gao X., Zhou C. Exploratory evaluation of EGFR-targeted anti-tumor drugs for lung cancer based on Lung-on-a-Chip. Biosensors. 2022;12(8):618. doi: 10.3390/bios12080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Kong N., Zhang X., Liu Y., Hu P., Mou S., Liljeström P., Shi J., Tan W., Kim J.S., Cao Y., Langer R., Leong K.W., Farokhzad O.C., Tao W. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020;5(11):847–860. doi: 10.1038/s41578-020-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T., Deng P., Wang Y., Zhang X., Guo Y., Chen W., Qin J. Microengineered multi-organoid system from hipscs to recapitulate human liver-islet axis in normal and type 2 diabetes. Adv. Sci. 2022;9(5) doi: 10.1002/advs.202103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatara A.M. Role of tissue engineering in COVID-19 and future viral outbreaks. Tissue Eng. Part A. 2020;26(9-10):468–474. doi: 10.1089/ten.TEA.2020.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker V.V., Sharma K., Dhar N., Mancini G.F., Sordet-Dessimoz J., Mckinney J.D. Rapid endotheliitis and vascular damage characterize SARS-CoV-2 infection in a human lung-on-chip model. EMBO Rep. 2021;22(6):e52744. doi: 10.15252/embr.202152744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li K., Xu G., Chen C., Song G., Dong Z., Lin L., Wang Y., Xu Z., Yu M., Yu X., Ying B., Fan Y., Chang L., Geng J. Low-Cost and scalable platform with multiplexed microwell array biochip for rapid diagnosis of COVID-19. Research (Wash D C). 2021;2021 doi: 10.34133/2021/2813643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Li E., Guo Z., Yu R., Hao H., Xu Y., Sun Z., Li X., Lyu J., Wang Q. Design and construction of a multi-organ microfluidic chip mimicking the in vivo microenvironment of lung cancer metastasis. Acs Appl. Mater. Inter. 2016;8(39):25840–25847. doi: 10.1021/acsami.6b08746. [DOI] [PubMed] [Google Scholar]
- Yang X., Li K., Zhang X., Liu C., Guo B., Wen W., Gao X. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip. 2018;18(3):486–495. doi: 10.1039/c7lc01224a. [DOI] [PubMed] [Google Scholar]
- Zamprogno P., Wuthrich S., Achenbach S., Thoma G., Stucki J.D., Hobi N., Schneider-Daum N., Lehr C.M., Huwer H., Geiser T., Schmid R.A., Guenat O.T. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun. Biol. 2021;4(1):168. doi: 10.1038/s42003-021-01695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wang P., Luo R., Wang Y., Li Z., Guo Y., Yao Y., Li M., Tao T., Chen W., Han J., Liu H., Cui K., Zhang X., Zheng Y., Qin J. Biomimetic human disease model of SARS-CoV-2-Induced lung injury and immune responses on organ chip system. Adv. Sci. (Weinh) 2021;8(3) doi: 10.1002/advs.202002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F., Jia J.L., Li L.M., Mao H.L., Zhou X.M., Luo H., Gao Y.F., Xu A.G. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.
No data was used for the research described in the article.