Table 1.
Optimization of the Reaction and Its Conditions a
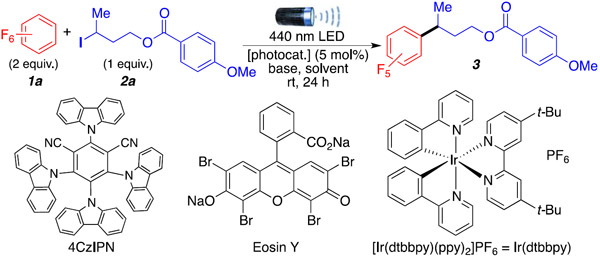
| ||||
|---|---|---|---|---|
| entry | base (equiv) | solvent (mL) | [photocatalyst] | yield (%)b |
| 1 | Et3N (2.0) | CH3CN (1.0) | 4CzIPN | 24 |
| 2 | Et3N (2.0) | DMSO (1.0) | 4CzIPN | 22 |
| 3 | Et3N (2.0) | 1,4-dioxane (1.0) | 4CzIPN | 26 |
| 4 | Et3N (2.0) | 1,4-dioxane (0.4) | 4CzIPN | 46 |
| 5 | DIPEA (2.0) | 1,4-dioxane (0.4) | 4CzIPN | 34 |
| 6 | DABCO (2.0) | 1,4-dioxane (0.4) | 4CzIPN | 0 |
| 7 | Et3N (3.0) | 1,4-dioxane (0.4) | 4CzIPN | 48 |
| 8 | Et3N (5.0) | 1,4-dioxane (0.4) | 4CzIPN | 50 |
| 9c | Et3N (5.0) | 1,4-dioxane (0.4) | 4CzIPN | 55 |
| 10d | Et3N (5.0) | 1,4-dioxane (0.4) | 4CzIPN | 62 (60)e |
| 11f | Et3N (5.0) | 1,4-dioxane (0.4) | 4CzIPN | 38 |
| 12 | – | 1,4-dioxane (0.4) | 4CzIPN | 0 |
| 13g | Et3N (5.0) | 1,4-dioxane (0.4) | 4CzIPN | 0 |
| 14 | Et3N (5.0) | 1,4-dioxane (0.4) | eosin Y | 38 |
| 15 | Et3N (5.0) | 1,4-dioxane (0.4) | Ir(dtbbpy) | 58 |
Optimal reaction conditions are as follows: 1 (2.0 mmol, 10 equiv), 2a (0.2 mmol, 1 equiv), base (1.0 mmol, 5 equiv), 1,4-dioxane (0.5 mL), 4CzIPN (5 mol %), 440 nm LED (40 W), room temperature (temperature around reaction flask was 35 °C due to heating caused by the LED lamp), reaction flask capped under argon, 24 h.
1H NMR yields using dibromomethane as internal standard.
1a (5 equiv) was used instead of 2 equiv.
1a (10 equiv) was used instead of 2 equiv.
Isolated yield.
A 427 nm LED (40W) was used instead of a 440 nm LED.
The reaction was performed in the dark.
