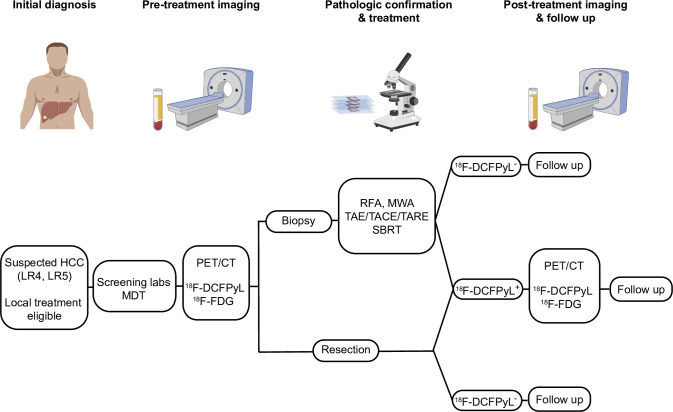
Fig 1. Protocol schema.
Patients who have LIRADS 4 or 5 lesions on standard imaging (CT or MRI) and deemed good candidates for any local treatment are eligible for this study. Prior to treatment, PET/CT with both 18F-DCFPyL and 18F-FDG will be performed. Histopathologic confirmation of HCC will be performed on either biopsy or surgical specimen. Only patients who had positive 18F-DCFPyL PET/CT pre-treatment will undergo post-treatment 18F-DCFPyL PET/CT (2–3 months post treatment) to determine its utility as a functional imaging marker. Scale bar shown is 100 μm. The images were created with BioRender.com, with permission privileges.