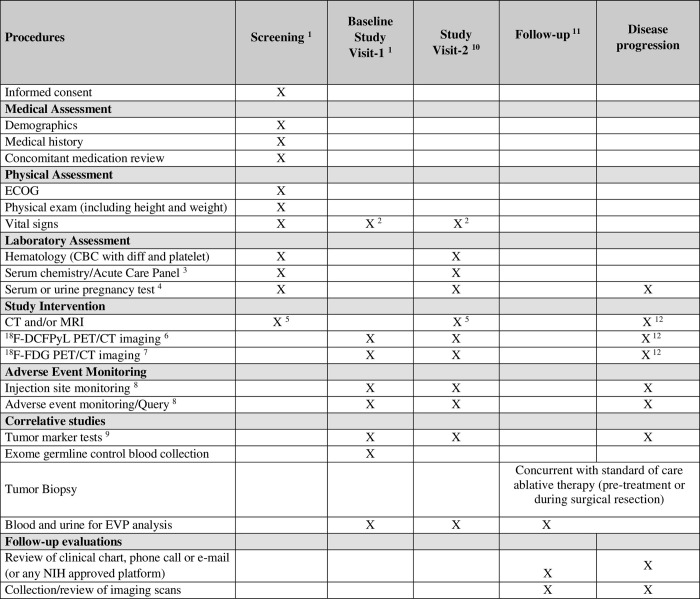
Fig 3. Study calendar.
Table numbers’ details: 1. Performed within 30 days prior to administration of 18F-DCFPyL unless otherwise indicated. Screening procedures such as medical assessment may be performed remotely via any NIH approved platforms. 2. Vital signs will be taken prior to injection of 18F-DCFPyL and following completion of the final PET/CT scan (+ 15 minutes). 3. Acute care panel: sodium, potassium, chloride, total CO2, creatinine, glucose, urea nitrogen, eGFR 4. For female participants of childbearing age (in the absence of prior hysterectomy). Pregnancy tests may be performed as clinically indicated prior to each scan. 5. CT (Chest/Abdomen/Pelvis) and/or MRI (Abdomen) will be performed within 2 months of each 18F-DCFPyL PET/CT. Imaging may be performed at certified outside facility and provided to study team. 6. Subjects will undergo 18F-DCFPyL injection and a dynamic PET/CT. Approximately 1 hour (+/- 10 minutes) post 18F-DCFPyL injection, a static PET/CT imaging performed. Refer to section 3.2.2 for additional information regarding the scanning procedure. 7. 18F-FDG PET/CT will be performed within approximately 2 weeks before or after each 18F-DCFPyL scan. Each PET/CT imaging must be approximately a day apart. The order obtained for 18F-DCFPyL PET/CT and 18F-FDG PET/CT does not matter. 8. Event monitoring will be done at the time of injection, and 1 hour post injection. All subjects will be contacted by phone at ~1–3 business days post-injection and will be asked non-leading questions regarding symptoms. At the investigator’s discretion, subjects with safety concerns noted during the post injection period may remain at the site or be asked to return to the site to undergo further safety assessments at the 1–3 business day follow-up time point. 9. Tumor markers include Alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 19.9 (CA19.9) and liver function tests (Alkaline Phosphatase, ALT, AST, Total Bilirubin, Direct Bilirubin). Refer to section 5.1 for more details. 10. Only participants with a positive baseline 18F-DCFPyL-PET/CT scan (i.e. with the presence of DCFPyL-avid tumor/s) and a biopsy confirming HCC diagnosis will undergo visit 2 for a second 18F-DCFPyL PET/CT (during routine treatment follow up, typically within 4–8 weeks). 11. Follow-up will be performed every 3 months after the last 18F-DCFPyL scan (or after therapy for participants with negative baseline 18F-DCFPyL PET/CT and biopsy confirming HCC) for 2 years, and yearly afterwards for an additional 3 years. 12. If tumor recurrence occurs during the follow-up period, an 18F-DCFPyL PET/CT, 18F-FDG PET/CT, CT/MRI and biopsy may be performed any time after recurrence at PI discretion. Biopsy would be performed concurrently with treatment if the latter is performed.