Abstract
Background
Cloperastine is a pivotal antibechic widely prescribed to treat cough caused by respiratory diseases. The present trial evaluated the pharmacokinetics (PK), bioequivalence (BE) and safety effects of the generic test (T) tablet of cloperastine after single-dose administration of cloperastine, compared with the original reference (R) tablet of cloperastine.
Objective
The purpose of this trial was to compare the PK, BE and safety of a test 10 mg versus the reference 10 mg formulation of cloperastine under fasting and postprandial conditions in healthy Chinese volunteers.
Methods
A single-centre, randomised, open, double-cycle, self-crossover, single oral administration Phase I trial was performed in healthy Chinese volunteers. A total of 60 subjects were enrolled in either the fasting (28 subjects) or the postprandial condition (32 subjects). Subjects randomly received a single dose of the T or R preparation (10 mg dose). Plasma concentrations of cloperastine were analysed by a validated LC-MS/MS method. The primary endpoints of the PK parameters were the area under the plasma concentration-time curve from zero to 72 h (AUC0–72h), under the plasma concentration-time curve from zero to infinity (AUC0–∞) and the maximal plasma concentration (Cmax). The equivalence standard range (80.0–125.0%) was used to evaluate the BE of the two preparations. The safety parameter as secondary endpoint was mainly evaluated by the occurrence of adverse events (AEs).
Results
A total of 25 and 30 subjects in the fasting and postprandial conditions completed this clinical trial, respectively. The geometric mean ratio (GMR) of the T/R for the Cmax, AUC0–72h and AUC0–∞ were 102.1%, 103.8% and 104.0% in the fasting condition, respectively. In the postprandial condition, the GMR of the T/R for the Cmax, AUC0–72h and AUC0–∞ were 94.2%, 98.8% and 99.0%, respectively. All the values fell within the range (80.0–125.0%). The Cmax and AUC0–72h values of the T and R preparations in fasting and postprandial conditions were not statistically significant (P > 0.05). Furthermore, no serious adverse events (SAEs) occurred during the whole trial.
Conclusions
The T and R preparations were bioequivalent under both conditions. Food has no significant effect on the absorption of cloperastine. Moreover, T and R preparations were well tolerated. The trial registration number (TRN) and date of registrations were CTR20212515, 13 October 2021.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40268-022-00406-2.
Key Points
| Bioequivalence between 10 mg test and reference cloperastine tablets was demonstrated following single dosing under fasting and postprandial conditions in healthy Chinese subjects. |
| The single 10 mg dose of cloperastine tablet was generally safe and well tolerated. |
| This test preparation of cloperastine could be a well-accepted alternative to the reference cloperastine. |
Introduction
Cough is a typical symptom of respiratory diseases, mainly caused by the stimulation of cough receptors located in the respiratory tract. Cough increases the risk of respiratory droplet transmission, and also reduces the quality of life of patients. Patients with cough caused by common viral infections such as COVID-19 and the flu virus need to take over-the-counter cough medicines [1]. It is estimated that the prevalence of chronic cough is as high as 13% of the general population [2, 3].
Cloperastine is a pivotal antitussive widely applied in clinical practice, mainly used for cough caused by respiratory diseases such as colds, acute bronchitis, chronic bronchitis, bronchiectasis, tuberculosis and lung cancer. Importantly, it not only suppresses the cough centre, but also inhibits G protein-coupled inwardly rectifying potassium (GIRK) channel-activated currents to loosen the cough [4]. Cloperastine has both H1-receptor blocking and antihistamine effects [5]. Studies have shown that it can improve the respiratory dysfunction of methyl CpG binding protein 2 (Mecp2) missing mice by enhancing the release of gamma-aminobutyric acid (GABA) [6]. Moreover, a previous study has shown that cloperastine was the preferred drug for relieving cough, it was much safer and more effective than codeine, and it did not inhibit respiration nor cause addiction. It also showed that coughing in children was alleviated after taking cloperastine [7, 8]. Recent studies have shown that cloperastine can also significantly inhibit the proliferation of oesophageal cancer cells by inhibiting mitochondrial oxidative phosphorylation [9]. Moreover, the researchers have found that cloperastine possesses antidepressant and antianxiety effects at the effective antitussive dose, and further discovered that it can reduce the damage of the passive avoidance response in mice exposed to diethylstilbestrol before delivery [4]. To date there has been little research on the safety and PK of cloperastine. Therefore, the clinical safety and PK characteristics of cloperastine need to be further evaluated to provide more research data on this drug.
The therapeutic dose of cloperastine is three times a day for adults, 10–20 mg each time. It takes effect after 20–30 min, and single-dose administration duration is 3–4 h [7]. After rapid oral absorption, it reaches peak concentration at 1–1.5 h, t1/2 was 23.0 ± 7.7 h, and AUC0–∞ was 81.0 ± 46.9 h∙ng/ml [10]. It is metabolised in the liver, and eliminated by the kidneys and bile within 24 h of administration [8].
This trial aimed to evaluate the PK, BE and safety aspects of two cloperastine preparations (produced by Dior Group Chengdu Pharmaceutical Co., Ltd., China and HUSTAZOL® Nipro ES Pharma Co., Ltd., respectively) after single-dose administration, and provide a reference for its approval of generic drugs in China.
Subjects and Methods
Study Design
A single-centre, randomised, open, double-cycle, self-crossover, single oral administration (10 mg) trial was conducted at the Phase I clinical research centre of Changsha Central Hospital from 28 June 2021 to 17 November 2021. The registration number of this trial was CTR20212515, registered on 13 October 2021 on the Drug Trial Registration and Information Publishing platform (http://www.chinadrugtrials.org.cn). It was approved by the Institutional Ethics Committee (Ethics approval No. [2021]010). All study participants signed informed consent forms.
The 28 and 32 subjects were randomly assigned to the fasting and postprandial conditions, respectively. The washout period was 7 days. After fasting over 10 h, the subjects in the fasting condition took T or R drug at random, while those in the postprandial condition took high-fat meals for 30 min before administration and then took them at random. Blood sample collection time was designed to occur near the maximum blood concentration range (1.5–4 h). After sample pretreatment, a rapid and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was used to determine the content of cloperastine for PK and BE analysis [11]. The main PK parameters were Cmax and AUC0–72h. The PK calculations were performed by WinNonlin 8.3. Safety evaluation was carried out by monitoring of vital signs, physical examination, electrocardiogram, laboratory examination and AE recording.
Subjects
According to the requirements of the Chinese pharmacopoeia, the number of subjects should not be less than 18. Therefore, 28 and 32 subjects were included in the fasting and postprandial conditions, respectively.
Subjects were required to satisfy all of the following criteria to be eligible for the trial: (1) Health Chinese subjects over 18 years of age, regardless of gender. (2) Male subjects weighing more than 50 kg, and female subjects were not to be less than 45 kg with a body mass index (BMI) that ranged from 19 kg/m2 to 26 kg/m2 (including boundary values). (3) Subjects who provided signed informed consent before the trial and fully understood the trial content, process and possible adverse reactions. (4) Subjects who communicated well with the researcher and understood and complied with the requirements of the trial.
In view of the drug safety, concomitant diseases, concomitant drugs, haemodynamics and other factors that may affect the subjects in the trial, and in reference to the drug manual of cloperastine, the exclusion criteria for subjects in this trial were formulated as follows—subjects meeting any of the following criteria were excluded: (1) Subjects who had participated in any other clinical trial within 3 months before the trial, who were allergic to cloperastine or diphenhydramine, or who had an allergic history. (2) Subjects with gastrointestinal diseases such as oesophageal disease, gastritis, gastric ulcer, enteritis, active gastrointestinal bleeding, gastrointestinal perforation or digestive tract surgery within the past 3 years. (3) Subjects with a history of disease of the cardiovascular system, endocrine system, nervous system, blood system or immune system (including individual or family history of hereditary immune deficiency), psychosis, metabolic abnormalities, malignant tumour, lymphoproliferative diseases, etc. (4) Subjects with orthostatic hypotension, fainting history or intolerance of venous puncture blood collection, or who had undergone surgery that affects drug absorption, distribution, metabolism and excretion within 6 months before the experiment. (5) Those who used any medicine (including healthcare products, Chinese herbal medicine, vitamins) for various reasons within the previous 14 days, and used drugs that inhibit or induce liver drug metabolism within the previous 30 days. (6) Blood donors or those who had lost a massive amount of blood (> 400 ml) within 3 months before the experiment. (7) Subjects receiving a blood transfusion or using blood products or planning to donate blood within 3 months. (8) Subjects who used cannabis within the previous 3 months or methylamphetamine, piperidine and other narcotics within the previous year, or who smoked more than five cigarettes per day within the 3 months before the trial. (9) Subjects who consumed overdoses of alcohol, tea, coffee and caffeine during the previous 6 month before the trial. (10) Those who were unable to abide by the unified diet or were lactose intolerant, had had unprotected sexual contact within 2 weeks before screening, or those who planned pregnancy or sperm donation or women who were pregnant or lactating within the 3 months before and after the trial. (11) Subjects who had abnormal results on vital signs, laboratory, ECG and physical examination.
Administration
The T and R preparations of 10 mg cloperastine tablets were produced by the Dior Group Chengdu Pharmaceutical Co., Ltd., China and HUSTAZOL® Nipro ES Pharma Co., Ltd., respectively.
The drug was administered on the morning of the trial in a fasting condition and drinking water was prohibited within 1 h before and after administration. The subjects in the postprandial condition were fed a high-fat and high-calorie breakfast after having blood taken on the morning of the day, and finished eating within 30 mins, then took the medicine with 240 ml water. Drinking water was also prohibited for 1 h before medication and 2 h after administration. The BE trial of the postprandial condition was carried out with food that had a strong influence on gastrointestinal physiological function and bioavailability of drugs (about 50% of the calories and 800–1,000 kcal high-fat diet). Subjects took standard meals after 4 h and 10 h of medication in the two conditions, and the second cycle repeated the meal schedule of the first cycle.
Collection and Preservation of Blood Samples
The t1/2 of cloperastine was 23.0 ± 7.7 h, the sampling time was to be not less than three terminal half-lives, and oral preparations with longer half-lives were to have a long enough biological sample collection time to cover the time of drug passing through the intestine and being absorbed. Therefore, blood was collected from 0 h to 72 h. The venous blood (4 ml) was collected in a heparin sodium anticoagulant tube before administration (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, 8, 10, 12, 24, 36, 48 h and 72 h after administration. After the blood samples of all subjects were collected, they were placed in a 2–8 °C centrifuge within 60 min after collection and centrifuged at 2000g for 10 min. The upper centrifuged plasma was divided into two parts, one for PK analysis and another for backup. The sample was immediately stored at − 80 °C after pretreatment for analysis.
Pharmacokinetic and Bioequivalence Analysis
Plasma concentrations of cloperastine were determined by Hunan Kerusi Pharmaceutical Technology Co., Ltd. using a validated LC-MS/MS method. The bioanalytical method and methodological validation results of cloperastine are given in the Online Supplementary Material (OSM) Resource 1 and Table S1. The PK analysis was performed with a non-compartment model using WinNonlin software 8.3 (Pharsight Corporation, Sunnyvale, CA, USA). The Cmax and Tmax values were acquired directly from concentration–time (C–T) curves of cloperastine. The AUC0−72h and AUC0–∞ were calculated using the linear trapezoidal rule, λz was the slope of the linear regression of the log-transformed C-T curve. Cloperastine plasma t1/2 was calculated as ln2/λz. The main PK indexes were Cmax, AUC0–72h and AUC0–∞, and the secondary PK parameters were Tmax, t1/2 and λz.
WinNonlin software 8.3 (Pharsight Corporation, Sunnyvale, CA, USA) was used to evaluate the BE of the main PK parameters. The Tmax values of the T and R preparations were statistically analysed by the Wilcoxon rank sum test. A multivariate analysis of variance (ANOVA) was performed after logarithmic transformation of the Cmax, AUC0–72h and AUC0–∞ of the two preparations. This trial also incorporated the inter-individual coefficients of variation (CV) into the BE evaluation compared with the previous studies. If the 90% confidence intervals (CIs) of the geometric mean ratio (GMR) for the main PK parameters of the T and R preparations were in the range of 80.0–125.0%, it was regarded as equivalent for the two preparations.
Safety
The AEs were recorded using the National Cancer Institute’s Common Adverse Event Terminology Standards (CTCAE) version 5.0. The evaluation criteria of AEs were mainly severity of reaction time, symptoms, signs and laboratory indicators. SAS software 9.4 (SAS Institute, Inc, Cary, NC, USA) was used to calculate baseline and safety data.
Results
Subjects Characteristics
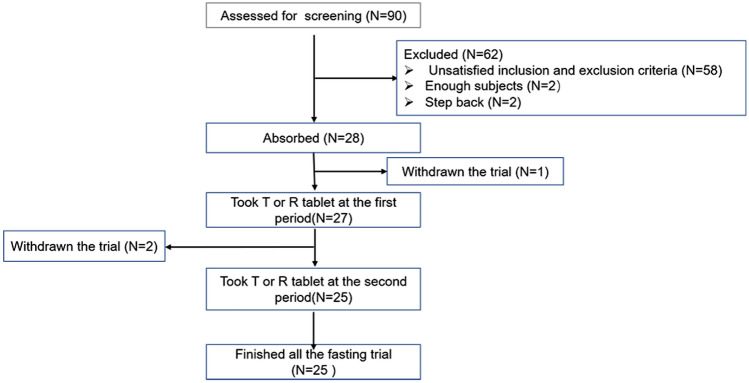
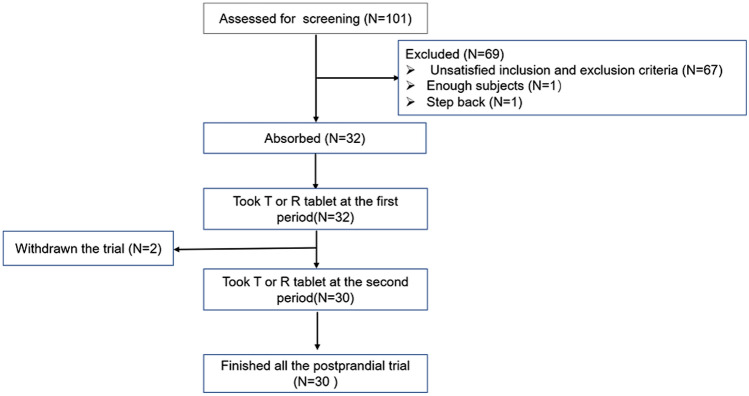
A total of 90 and 101 subjects were screened in the fasting and postprandial conditions, respectively, and 28 subjects (25 males and three females) and 32 subjects (29 males and three females) without a difference in sex ratio (P > 0.05) in the two conditions completed the trial, respectively. Out of the entire population, three and two subjects in the fasting and postprandial conditions, respectively, refused to participate in the trial. The screening and inclusion distributions of subjects in the two conditions are shown in Figs. 1 and 2. The age ranges of the participants in the two conditions were 18–39 and 18–43 years, respectively. The ranges of body weight, height and BMI of the fasting and postprandial conditions were 45.0–76.5 kg and 52.9–73.2 kg, 146.0–178.0 cm and 150.0–175.0 cm, and 19.0–24.7 kg/m2 and 19.4–26.0 kg/m2, respectively. The baseline characteristics of each condition are shown in Table 1.
Fig. 1.
Flow chart of subject distribution under the fasting condition. T test preparation: Dior Group Chengdu Pharmaceutical Co., Ltd.; R reference preparation: HUSTAZOL® Nipro ES Pharma Co., Ltd.
Fig. 2.
Flow chart of subject distribution under the postprandial condition. T test preparation: Dior Group Chengdu Pharmaceutical Co., Ltd.; R reference preparation: HUSTAZOL® Nipro ES Pharma Co., Ltd.
Table 1.
The baseline characteristics for the fasting and postprandial conditions
| Parameters | Fasting group (N = 28) | Postprandial group (N = 28) |
|---|---|---|
| Gender n (%) | ||
| Male | 25 (89.3 %) | 29 (90.6 %) |
| Female | 3 (10.7 %) | 3 (9.4 %) |
| Age (y) | ||
| Mean ± SD | 25.2 ± 6.4 | 25.0 ± 6.4 |
| Min–max | 18–39 | 18–43 |
| Height (cm) | ||
| Mean ± SD | 165.8 ± 8.4 | 166.6 ± 6.0 |
| Min–max | 146.0–178.0 | 150.0–175.0 |
| Weight (kg) | ||
| Mean ± SD | 60.4 ± 8.1 | 61.7 ± 5.0 |
| Min–max | 45.0–76.5 | 52.9–73.2 |
| BMI (kg/m2) | ||
| Mean ± SD | 21.9 ± 1.9 | 22.2 ± 1.7 |
| Min–max | 19.0–24.7 | 19.4–26.0 |
BMI body mass index, Max maximum, Min minimum
Pharmacokinetic and Bioequivalence
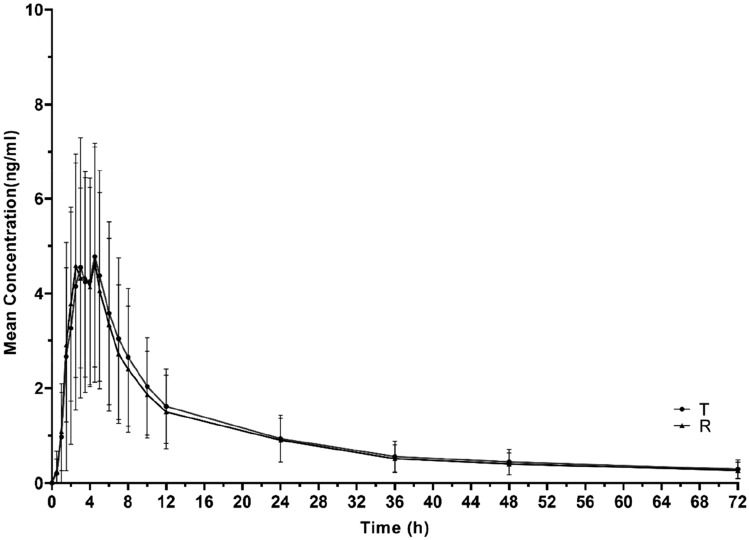
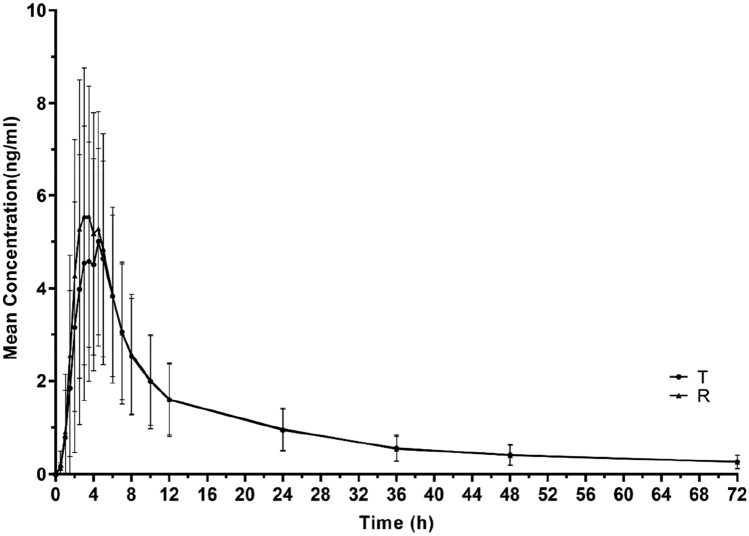
The PK parameters under fasting and postprandial conditions are summarised in Table 2. Under the two conditions, the mean plasma concentration-time curve of a single dose of cloperastine and their logarithmic figures are shown in Figs. 3 and 4. In the fasting condition, the mean values of the Cmax for the T and R tablets were 5.3 and 5.2 ng/ml, and those of the AUC0–72h were 72.4 and 68.6 ng∙h/ml, respectively. The mean values of the AUC0–∞ for the T and R tablets were 88.4 and 83.8 ng∙h/ml, respectively. In the postprandial condition, the average values of the Cmax for the T and R tablets were 6.8 and 7.2 ng/ml, those for AUC0–72h were 73.2 and 73.9 ng∙h/ml, and those for AUC0–∞ were 87.4 and 87.7 ng∙h/ml, respectively. Tmax values for the two preparations were 4.0 and 3.5 h, and 3.0 and 2.8 h in the fasting and postprandial conditions, respectively. Similarly, t1/2 of the T and R tablets was approximately 35 h under fasting and postprandial conditions.
Table 2.
The PK parameters of cloperastine preparations after a single oral (10 mg) dose under fasting and postprandial conditions
| PK parameter | Fasting | Postprandial | ||
|---|---|---|---|---|
| Mean ± SD (CV %) | Mean ± SD (CV %) | |||
| T (N = 25) | R (N = 27) | T (N = 30) | R (N = 32) | |
| Tmax (h) | 4.0 (1.5,5.0) | 3.5 (1.5,4.5) | 3.0 (1.5,5.0) | 2.8 (1.5,5.0) |
| Cmax (ng/mL) | 5.3 ± 2.8 (53.0 %) | 5.2 ± 2.7 (52.1 %) | 6.8 ± 3.0 (43.9 %) | 7.2 ± 3.1 (43.3 %) |
| AUC0–72h (h∙ng/mL) | 72.4 ± 38.1 (52.7 %) | 68.6 ± 34.5 (50.3 %) | 73.2 ± 39.4 (53.9 %) | 73.9 ± 43.6 (59.0 %) |
| AUC0–∞ (h∙ng/mL) | 88.4 ± 48.8 (55.2%) | 83.8 ± 46.4 (55.4%) | 87.4 ± 52.9 (60.6%) | 87.7 ± 58.1 (66.3%) |
| λz | 0 (19.7 %) | 0 (28.0 %) | 0 (24.8 %) | 0 (19.4 %) |
| t1/2 (h) | 35.8 ± 7.8 (21.7 %) | 35.5 ± 9.1 (25.5 %) | 34.9 ± 9.2 (26.4 %) | 34.9 ± 6.7 (19.2 %) |
T test preparation, Dior Group Chengdu Pharmaceutical Co., Ltd., R reference preparation, HUSTAZOL® Nipro ES Pharma Co., Ltd.
Fig. 3.
The concentration-time curves of T and R under the fasting condition (NT = 25, NR = 27). T test preparation: Dior Group Chengdu Pharmaceutical Co., Ltd.; R reference preparation: HUSTAZOL® Nipro ES Pharma Co., Ltd.
Fig. 4.
The concentration-time curves of T and R under the postprandial condition (NT = 30, NR = 32). T test preparation: Dior Group Chengdu Pharmaceutical Co., Ltd.; R reference preparation: HUSTAZOL® Nipro ES Pharma Co., Ltd.
The BE evaluations between the T and R preparations of cloperastine 10 mg tablets in healthy subjects under fasting and postprandial conditions are presented in Table 3. The results indicate that the GMR of T/R for the Cmax, AUC0–72h and AUC0–∞ were 102.1%, 103.8% and 104.0%, respectively. In the postprandial condition, the GMR values of T/R for the Cmax, AUC0–72h and AUC0–∞ were 94.2%, 98.8% and 99.0%, respectively. All the values were fell within the range of 80.0–125.0%. Therefore, the T and R preparations were considered to be equivalent.
Table 3.
The BE assessments under fasting and postprandial conditions
| Condition | PK parameter | Preparation | GMR (%) | CV (%) | 90 % CI (%) | |
|---|---|---|---|---|---|---|
| T | R | |||||
| Fasting (NT = 24, NR = 26) | Cmax (ng/ml) | 4.8 | 4.7 | 102.1 | 17.5 | 93.6–111.2 |
| AUC0–72h (h∙ng/ml) | 63.7 | 61.3 | 103.8 | 16.4 | 95.7–112.5 | |
| AUC0–∞ (h∙ng/ml) | 75.9 | 73.0 | 104.0 | 16.2 | 96.0–112.7 | |
| Postprandial (NT = 30, NR = 32) | Cmax (ng/ml) | 6.2 | 6.6 | 94.2 | 17.1 | 87.4–101.5 |
| AUC0–72h (h∙ng/ml) | 65.4 | 66.2 | 98.8 | 12.5 | 93.5–104.3 | |
| AUC0–∞ (h∙ng/ml) | 76.3 | 77.1 | 99.0 | 13.1 | 93.5–104.8 | |
GMR the geometric mean ratio of T over R PK metric, CV intraindividual variation, T test preparation, Dior Group Chengdu Pharmaceutical Co., Ltd., R reference preparation, HUSTAZOL® Nipro ES Pharma Co., Ltd.
ANOVA was used to analyse the PK parameters under both conditions; results are shown in Table S2 (OSM). The results show that the period factor was observed for the PK parameters in the two conditions.
Safety and Tolerability
In the fasting (27 subjects) and postprandial (32 subjects) conditions, 41 AEs occurred in 18 subjects (66.7%) and 20 AEs occurred in 12 subjects (60.0%), respectively. Among the subjects taking the T or R preparations, there were 24 AEs (52.0%) in 13 subjects and 17 AEs (44.4%) in 12 subjects. There were no adverse drug reactions (ADRs) and SAEs in the fasting and postprandial conditions in this trial. The correlation between AEs and drug administration was stratified into five levels, i.e., definitely related, probably related, possibly related, possibly unrelated and definitely unrelated, and the following correlations were considered as ADRs: definitely related, probably related and possibly related, which did not occur in this trial, and all AEs were possibly unrelated to the drugs throughout the whole trial process. Moreover, AEs of 16 males and two females occurred in the fasting condition, and that of ten males and two females in the postprandial condition, respectively. However, these AEs belong to grade 1, and no SAEs occurred in males or females in this trial. A summary of AEs during the trial are shown in Table 4.
Table 4.
Summary of AEs under the fasting and postprandial conditions
| Parameter | Fasting group | Postprandial group | ||||
|---|---|---|---|---|---|---|
| T (N = 25) n (%) E | R (N = 27) n (%) E | Total n (%) E | T (N = 30) n (%) E | R (N = 32) n (%) E | Total n (%) E | |
| Sum | 13 (52.0) 24 | 12 (44.4) 17 | 18 (66.7) 41 | 6 (20.0) 9 | 7 (21.9) 11 | 12 (37.5) 20 |
| AE severity | ||||||
| Grade 1 | 13 (52.0) 24 | 12 (44.4) 17 | 25 (48.1) 41 | 6 (20.0) 9 | 7 (21.9) 11 | 13 (21.0) 20 |
| ≥ Grade 2 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| No. of subjects discontinuing due to AEs | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Correlation with drugs | ||||||
| Possible unrelated | 13 (52.0) 24 | 12 (44.4) 17 | 25 (48.1) 41 | 6 (20.0) 9 | 7 (21.9) 11 | 13 (21.0) 20 |
| AEs | ||||||
| A/G increased | 1 (4.0) 1 | 2 (7.4) 2 | 3 (11.1) 3 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| ALT elevated | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| WBC (+) | 2 (8.0) 4 | 1(3.7) 2 | 3 (11.1) 6 | 1 (3.3) 1 | 1 (3.1) 1 | 2 (6.3) 2 |
| RBC (+) | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 1 (3.3) 1 | 0 (0) 0 | 1 (3.1) 1 |
| BLD (+) | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 1 (3.3) 1 | 1 (3.3) 1 | 2 (6.3) 2 |
| GLU | 1 (4.0) 1 | 0 (0) 0 | 1(3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| NIT | 0 (0) 0 | 1 (3.7) 1 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| BASO% increased | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| BASO# increased | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Heart rate decreased | 0 (0) 0 | 1 (3.7) 1 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Haemobilirubin increased | 0 (0) 0 | 1 (3.7) 1 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| UCB elevated | 1 (4.0) 1 | 1 (3.7) 1 | 2 (7.4) 2 | 1 (3.3) 1 | 0 (0) 0 | 1 (3.1) 1 |
| SCR elevated | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Uric acid elevated | 1 (4.0) 1 | 1 (3.7) 1 | 2 (7.4) 2 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Fibrinogen decreased | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 1 (3.3) 1 | 0 (0) 0 | 1 (3.1) 1 |
| Blood pressure decreased | 3 (12.0) 3 | 2 (7.4) 2 | 5 (18.5) 5 | 1 (3.3) 1 | 1 (3.1) 1 | 2 (6.3) 2 |
| Blood pressure increased | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 1 (3.3) 1 | 0 (0) 0 | 1 (3.1) 1 |
| Neutrophil count decreased | 0 (0) 0 | 1 (3.7) 1 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Skin scratch | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Chest discomfort | 0 (0) 0 | 1 (3.7) 1 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Diarrhoea | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Ventricular extrasystole | 0 (0) 0 | 1 (3.7) 1 | 1 (3.7) 1 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Sinus bradycardia | 1 (4.0) 1 | 0 (0) 0 | 1 (3.7) 1 | 1 (3.3) 1 | 1 (3.1) 1 | 2 (6.3) 2 |
| Lymphadenopathy | 1 (4.0) 1 | 1 (3.7) 1 | 1 (3.7) 2 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 |
| Hyperuricacidemia | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 1 (3.1) 1 | 1 (3.1) 1 |
| PR of ECG shortened | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 1 (3.13) 1 | 1 (3.13) 1 |
| Abnormal ECG repolarization | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 1 (3.1) 1 | 1 (3.1) 1 |
| Serum creatine phosphokinase MB increased | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 1 (3.1) 1 | 1 (3.1) 1 |
| Serum creatine phosphokinase increased | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 1 (3.1) 1 | 1 (3.1) 1 |
| Anemia | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 0 (0) 0 | 1 (3.1) 1 | 1 (3.1) 1 |
% percentage based on N, N number of subjects, n number of subjects with AEs, E number of AEs, AEs adverse events, A/G albumin/globulin, ALT alanine transaminase, WBC white blood cell, RBC red blood cell, BLD blood, GLU glucose in urine, NIT nitrite, BASO% basophil ratio, BASO# basophil count, UCB unconjugated bilirubin, SCR serum creatinine, T test preparation, Dior Group Chengdu Pharmaceutical Co., Ltd., R reference preparation, HUSTAZOL® Nipro ES Pharma Co., Ltd.
Discussion
Cough is a reflex activity when the respiratory system is stimulated, which is often caused by a cold. Cloperastine is a non-addictive central antitussive, which is safer than opioid central antitussive drugs with fewer side effects [12]. Moreover, previous studies of cloperastine have mainly evaluated its efficacy, and there is a lack of the human studies for the PK and safety of this drug, which has limited its clinical application [13]. Therefore, this study evaluated the PK of cloperastine tablets in healthy Chinese subjects to better understand its in vivo characteristics. The BE between the generic preparation produced by Dior Group Chengdu Pharmaceutical Co., Ltd., China and the original preparation was also evaluated to provide a reference for its approval as a generic drug in China [14–16].
In addition, an appropriate gender ratio was also considered in this trial. However, it was generally recognised that the gender imbalances in previous BE studies were due to the provisions on gender ratio in BE research lacking international unified standards [17, 18]. In some countries, BE clinical trials do not include female subjects, while the guidelines for BE clinical trials in China require an appropriate proportion of female subjects. However, there was no specific requirement for the numbers of female subjects in the Chinese BE clinical trials [16]. Therefore, this trial included a larger percentage of men and a nominal percentage of women. The effect of gender differences on the efficacy of cloperastine needs to be investigated in further clinical trials.
In this trial, the T preparation in the fasting and postprandial conditions reached Tmax at 4.0 h and 3.0 h, respectively. Under the postprandial condition, the absorption rate of the drugs decreased, and food may affect the absorption rate. Under the fasting condition, the Cmax values of the T and R preparations were 5.3 ng/ml and 5.2 ng/ml, respectively. The AUC0–72h values of the two preparations were 72.4 h∙ng/ml and 68.6 h∙ng/ml, respectively. It was concluded that the bioavailability of the T preparation was higher than that of the R preparation under fasting conditions. Under fasting and postprandial conditions, all values were within 80.0–125.0%, which could infer that the two preparations were equivalent.
After oral administration, physical or chemical reactions between food and drugs affect the absorption of drugs. Food will also change the pH value of the gastrointestinal tract and the rate of gastric emptying, reducing drug absorption. But food had no significant effect on the absorption of cloperastine in our study (P > 0.05).
The CV value of cloperastine was unknown in previous studies, but the subjects showed certain variability in the trial. The CV values of the Cmax and AUC0–72h in the fasting condition were 17.5% and 16.4%, respectively, while those in the postprandial condition were 17.1% and 12.5%, respectively. The reasons for variation may be associated with physiological characteristics and genetic polymorphisms of the drug metabolism gene of subjects, which needs further study. The ANOVA analysis of the PK parameters showed that the periodic factors were statistically significant. Of note, under the fasting and postprandial conditions, during the two periods, the individual PK parameters of the same subject can be affected by mixed factors such as sleep quality, exercise activity and mental state, thus causing significant differences in PK parameters such as Cmax and AUC during the two periods. This trial also included safety assessment; no ADRs or SAEs occurred throughout the clinical trial, which was consistent with the results of safety studies on cloperastine [7]. This result indicates that 10 mg cloperastine is safe for clinical use.
Finally, most drugs are found with the discovery of targets. The targets are widely used to explain therapeutic effects and side effects. However, cloperastine is one of the seven globally approved drugs that have no known molecular therapeutic targets. It was first discovered in 1961. Its histamine activity can explain the occurrence of its side effects, but it does not confirm the mechanism involved in cough treatment. Later studies have confirmed that the targets of cloperastine are the σ receptor, histamine H1 receptor and histamine H3 receptor [19, 20]. However, the association of these targets with therapeutic efficacy and safety needs to be further studied. In addition, its metabolic enzymes are still unidentified, which also limits pharmacogenetic research and more accurate individualised treatment.
Conclusion
This trial has shown that the generic T tablet of 10 mg cloperastine was bioequivalent to the original R tablet under both fasting and postprandial conditions, which meets the requirements of BE. Food has no significant effect on the absorption of cloperastine. Moreover, both preparations showed good tolerability and safety.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The work was supported by the Phase I Clinical Trial Centre, Changsha Central Hospital affiliated to University of South China, Changsha, Hunan. The authors thank Professor Gao for his guidance in writing this paper, and thank all the researchers and nurses for their support and dedication.
Author Contributions
All authors made substantial contributions in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for the work.
Declarations
Data availability statement
All data generated or analysed during this study are included in this published article.
Consent to participate
Not applicable .
Consent for publicaiton
Not applicable .
Code availability
Not applicable.
Conflict of interest
The authors declare no conflicts of interest in this work.
Ethics statement
The study involving human participants was registered on the Platform for Bioequivalence and Clinical Trials registration prior to the trial and approved by the Ethics Committee of Changsha Central Hospital affiliated to the University of South China. All subjects provided informed consent.
Funding
This work was supported by the Science and Technology Key Program of Hunan Province Grants (2016SK2066), Hunan Province Chinese Medicine Research Program Grants (201940), Science and Technology Key Program of Hunan Provincial Health Committee (20201904), Natural Science Foundation of Hunan Province (2021JJ30753), Changsha Central Hospital Affiliated to University of South China Foundation of key Program (YNKY202205), and Hunan Province Foundation of High-level Health Talent (225 Program).
References
- 1.Song W-J, Hui CKM, Hull JH, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9(5):533–544. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visca D, Beghe B, Fabbri LM, et al. Management of chronic refractory cough in adults. Eur J Intern Med. 2020;81:15–21. doi: 10.1016/j.ejim.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(1):1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soeda F, Hirakawa E, Inoue M, et al. Cloperastine rescues impairment of passive avoidance response in mice prenatally exposed to diethylstilbestrol. Environ Toxicol. 2014;29(2):216–225. doi: 10.1002/tox.21749. [DOI] [PubMed] [Google Scholar]
- 5.Lun J, Zhao P, Jiang Z, et al. Enantioselective LC-MS/MS method for the determination of cloperastine enantiomers in rat plasma and its pharmacokinetic application. Chirality. 2020;32(8):1129–1138. doi: 10.1002/chir.23257. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CM, Cui N, Xing H, et al. The antitussive cloperastine improves breathing abnormalities in a Rett Syndrome mouse model by blocking presynaptic GIRK channels and enhancing GABA release. Neuropharmacology. 2020;176:108214. doi: 10.1016/j.neuropharm.2020.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catania MA, Cuzzocrea S. Pharmacological and clinical overview of cloperastine in treatment of cough. Ther Clin Risk Manag. 2011;7:83–92. doi: 10.2147/TCRM.S16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaura K, Ogata Y, Inoue M, et al. The centrally acting non-narcotic antitussive tipepidine produces antidepressant-like effect in the forced swimming test in rats. Behav Brain Res. 2009;205(1):315–318. doi: 10.1016/j.bbr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Yu Y, Jiang Y, et al. Cloperastine inhibits esophageal squamous cell carcinoma proliferation in vivo and in vitro by suppressing mitochondrial oxidative phosphorylation. Cell Death Discov. 2021;7(1):166. doi: 10.1038/s41420-021-00509-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Zhang C, Wang J, et al. Simultaneous quantitation of paracetamol, caffeine, pseudoephedrine, chlorpheniramine and cloperastine in human plasma by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010;51(3):716–722. doi: 10.1016/j.jpba.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Segovia G, Del Arco A, De Blas M, et al. Environmental enrichment increases the in vivo extracellular concentration of dopamine in the nucleus accumbens: a microdialysis study. J Neural Transm (Vienna) 2010;117(10):1123–1130. doi: 10.1007/s00702-010-0447-y. [DOI] [PubMed] [Google Scholar]
- 12.Serrano M, Sanz-Cuesta M, Villaronga M, et al. Cloperastine-based cough syrup and acute dystonic reactions. Dev Med Child Neurol. 2012;54(3):287. doi: 10.1111/j.1469-8749.2011.04105.x. [DOI] [PubMed] [Google Scholar]
- 13.Takahara A, Fujiwara K, Ohtsuki A, et al. Effects of the antitussive drug cloperastine on ventricular repolarization in halothane-anesthetized guinea pigs. J Pharmacol Sci. 2012;120(3):165–175. doi: 10.1254/jphs.12117FP. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Qi L, Bai H, et al. Pharmacokinetics and bioequivalence of rasagiline tablets in chinese healthy subjects under fasting and fed conditions: an open, randomized, single-dose, double-cycle, two-sequence, crossover trial. Front Pharmacol. 2020;11:571747. doi: 10.3389/fphar.2020.571747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi A, Kiang YH, Saw RE, et al. Evaluation of the pharmacokinetics and safety of AMG 986 tablet and capsule formulations in healthy adult subjects: a phase I, open-label, randomized study. Drugs R D. 2022;22(2):147–154. doi: 10.1007/s40268-022-00388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun ML, Liu HJ, Luo XD, et al. Bioequivalence and safety assessment of two formulations of metformin hydrochloride sustained-release tablets (Yuantang((R)) SR and Glucophage((R)) XR) under fed conditions in healthy Chinese adult subjects: an open-label, two-way crossover, sequence randomized phase I clinical trial. Drugs R D. 2022;22(1):51–60. doi: 10.1007/s40268-021-00377-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alloway RR, Vinks AA, Fukuda T, et al. Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: a randomized, crossover clinical trial. PLoS Med. 2017;14(11):e1002428. doi: 10.1371/journal.pmed.1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Wang X, Chen Q, et al. Pharmacokinetics and bioequivalence of two formulations of valsartan 80 mg capsules: a randomized, single dose, 4-period crossover study in healthy chinese volunteers under fasting and fed conditions. Drug Des Dev Ther. 2020;14:4221–4230. doi: 10.2147/DDDT.S253078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregori-Puigjane E, Setola V, Hert J, et al. Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci USA. 2012;109(28):11178–11183. doi: 10.1073/pnas.1204524109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Civardi C, Collini A, Enrico E, et al. Induced hemichorea by cloperastine overuse. Neurol Sci. 2020;41(7):1959–1960. doi: 10.1007/s10072-020-04267-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.