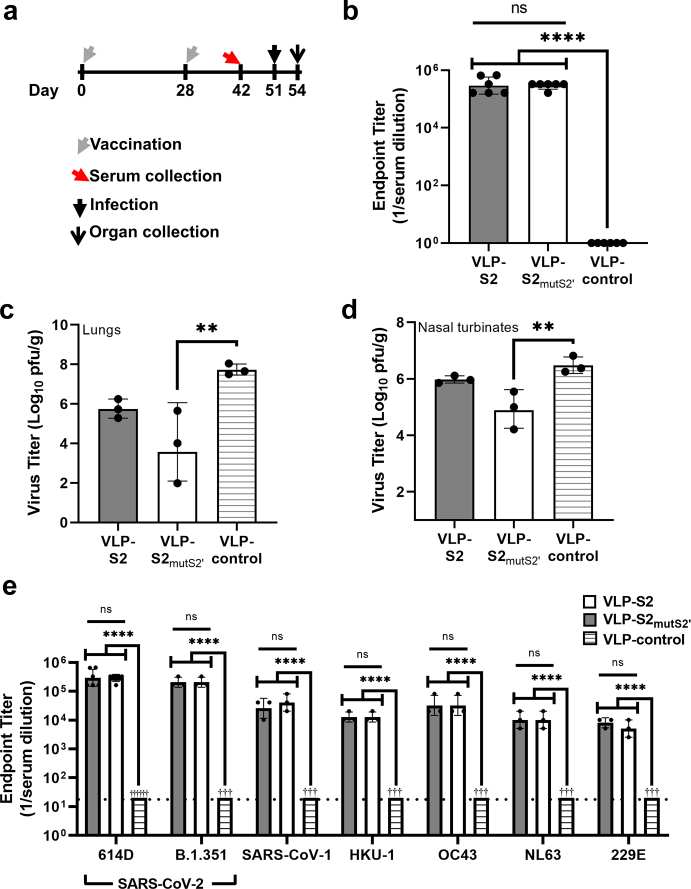
Fig. 4.
Characterization of the efficacy of VLP-S2 and VLP-S2mutS2’ in hamsters.(a) Schedule for hamster vaccination, serum collection, infection with SARS-CoV-2, and organ collection. (b) Antibody endpoint titers of sera from hamsters immunized with either VLP-S2, VLP-S2mutS2, or VLP-control against SARS-CoV-2 spike protein (geometric mean with geometric SD, n = 6, biological replicates = 3: two independent assays with sera from 3 hamsters). ns: not statistically significant, ∗∗∗∗P < 0.0001 [one-way analysis of variance (ANOVA) and Tukey post-hoc multiple comparison between groups (α = 0.05)]. (c) Viral titer in the lungs of hamsters immunized with either VLP-S2, or VLP-S2mutS2’, or VLP-control three days after infection with SARS-CoV-2 (mean with SD, n = 3, biological replicates = 3: tissues from 3 hamsters). ∗∗P = 0.0098 [one-way analysis of variance (ANOVA) and Dunnett post-hoc multiple comparison between groups (α = 0.05)]. (d) Viral titer in the nasal turbinates of hamsters immunized with either VLP-S2, or VLP-S2mutS2, or VLP-control three days after SARS-CoV-2 infection (mean with SD, n = 3, biological replicates = 3: tissues from 3 hamsters). ∗∗P = 0.0057 [one-way analysis of variance (ANOVA) and Dunnett post-hoc multiple comparison between groups (α = 0.05)]. (e) Antibody endpoint titers of sera from hamsters immunized with either VLP-control, VLP-S2 (gray), or VLP-S2mutS2 (white) against spike proteins of the original Wuhan-Hu-1 SARS-CoV-2 (614D), the SARS-CoV-2 variant B.1.351, SARS-CoV-1, and the four endemic human coronaviruses HKU-1, OC43, NL63, and 229E (geometric mean with geometric SD, n = 6, biological replicates = 3 against SARS-CoV-2 614D S protein: two independent assays with sera from 3 hamsters; n = 3, biological replicates = 3 against all other S proteins: sera from 3 hamsters). ns: not statistically significant, ∗∗∗∗P < 0.0001 [one-way analysis of variance (ANOVA) and Tukey post-hoc multiple comparison between groups (α = 0.05)].