Abstract
Schistosomiasis, a parasitic disease caused by the blood fluke of the genus Schistosoma, affects over 230 million people, especially in developing countries. Despite the significant economic and public health consequences, only one drug is currently available for treatment of schistosomiasis, praziquantel. Thus, there is an urgent demand for new anthelmintic agents. Based on our continuous studies involving the chemical prospection of floristic biodiversity aiming to discover new bioactive compounds, this work reports the in vitro antiparasitic activity against Schistosoma mansoni adult worms of neolignans threo-austrobailignan-6 and verrucosin, both isolated from Saururus cernuus L. (Saururaceae). These neolignans showed a significant in vitro schistosomicidal activity, with EC50 values of 12.6–28.1 µM. Further analysis revealed a pronounced reduction in the number of S. mansoni eggs. Scanning electron microscopy analysis revealed morphological alterations when schistosomes were exposed to either threo-austrobailignan-6 or verrucosin. These relevant antischistosomal properties were accompanied by low cytotoxicity potential against the animal (Vero) and human (HaCaT) cell lines, resulting in a high selectivity index. Considering the promising chemical and biological properties of threo-austrobailignan-6 and verrucosin, this research should be of interest to those in the area of neglected diseases and in particular antischistosomal drug discovery.
Subject terms: Antiparasitic agents, Parasitology
Introduction
Schistosomiasis, a poverty-associated parasitic disease caused by blood fluke of the genus Schistosoma, is a debilitating disease with a tremendous global burden. Estimates show that over 230 million people are affected with schistosomiasis in 78 countries and approximately 10% of the world population is at risk for infection1. Schistosoma mansoni, one of the three major human species, occurs across much of Africa, the Middle East, the Caribbean, and South America. Morbidity due to schistosomiasis mansoni includes hepatosplenomegaly, liver fibrosis, and ascites; in severe cases, S. mansoni infection can be fatal2. Consequently, it is a disease of immense medical importance.
The control of schistosomiasis is mainly dependent on the use of praziquantel, the only readily commercially available drug3,4. The Word Health Organization (WHO) strategy for schistosomiasis control focuses on large-scale treatment (preventive chemotherapy) of affected populations, a strategy that might select for drug-resistant parasites. In addition, numerous persistent schistosomiasis hotspots remain5,6, and low cure rates have been reported7,8. Systematic reviews and meta-analyses show that praziquantel achieves a cure rate of approximately 75% for S. mansoni infections9,10, demonstrating the limitations of praziquantel. With the aim to eliminate human schistosomiasis as a public health problem by 2030, in their new road map for neglected tropical diseases 2021–2030, the WHO highlights the need for new therapeutic interventions11. Thus, the pressing need to develop new anthelmintic compounds has been emphasized and the drug discovery landscape has gained momentum over the past few years12–14.
Saururus cernuus L. (S. cernuus, Saururaceae) is a type of freshwater plant widely distributed in America, including Brazil15. In folk medicine, this plant has been used as an anti-inflammatory and as a sedative15,16. Phytochemically, S. cernuus produces mainly lignoids but the occurrence of other compounds such as alkaloids and terpenoids was also reported17–19. Studies describing antiprotozoal effects of some lignoids isolated from S. cernuus were previously described, including threo,threo- and threo,erythro-manassantin A with activity against amastigote forms of Leishmania amazonensis20 and threo-austrobailignan-5, threo-austrobailignan-6, and threo-dihydroguaiaretic acid with activity against trypomastigote and amastigote forms of Trypanosoma cruzi21. Furthermore, alterations in the plasma membrane permeability, in reactive oxygen species (ROS) levels, and mitochondrial membrane potential caused by these compounds in tested parasites were observed. More recently, the molecular dereplication and the evaluation of anti-T. cruzi activity of volatile oils from inflorescences, leaves, branches, and roots of S. cernuus was reported. As described, the predominance of mono and sesquiterpenes was observed, as well as phenylpropanoids as the main compounds. Interestingly, oils from leaves, branches, inflorescences, and roots displayed activity against trypomastigotes, with reduced toxicity against NCTC cells22. This selectivity makes these compounds attractive as antiparasitic agents.
As part of our continuous study aiming to discover new antischistosomal agents from plants of Brazilian biodiversity23–25, this study reports the isolation of two neolignans from leaves of S. cernuus, of which one is dibenzylbutane—threo-austrobailignan-6—and one tetrahydrofuran—verrucosin—and their evaluation of anthelmintic properties against S. mansoni was performed, since these compounds exhibited activity against other parasites such as T. cruzi and L. infantum.
Results and discussion
Chemical characterization of threo-austrobailignan-6 and verrucosin
NMR spectral data of threo-austrobailignan-6 were identical to those reported in the literature26. As observed, the 1H NMR spectrum indicated the presence of two 1,3,4-trissubtituted aromatic rings due the signals at δ 6.67 (d, J = 1.7 Hz, H-2), 6.70 (d, J = 7.8 Hz, H-5), 6.60 (d, J = 1.7 Hz, H-2′), 6.80 (d, J = 7.9 Hz, H-5′), and 6.57–6.53 (m, H-6 and H-6′). The presence of four double-doublets at δ 2.39 (J = 13.5 and 8.0 Hz), 2.57 (J = 13.5 and 6.5 Hz), 2.33 (J = 13.5 and 8.0 Hz), and 2.56 (J = 13.5 and 6.5 Hz), assigned to H-7a, H-7b, H-7′a and H-7′b, respectively, in association with two doublets at δ 0.86 (J = 5.9, H-9) and 0.81 (J = 6.6, H-9′), indicated the occurrence of a dibenzylbutane neolignan21. Two singlets at δ 5.93 (2H) and 3.84 (3H) indicated the presence of methylenedioxy and methoxy groups, respectively, in the aromatic rings. 13C NMR spectrum of shown signals referring to aromatic carbons from δ 107.9 to 147.3 (C-1 to C-6 and C-1′ to C-6′), two methylene carbons at δ 41.1 (C-7) and 41.0 (C-7′), two methine carbons at δ 37.9 (C-8) and 37.8 (C-8′), and two methyl groups at δ 13.9 (C-9) and 13.8 (C-9′). Furthermore, signals at δ 100.9 and 55.8 were assigned to methylenedioxy and methoxy groups, respectively. Finally, based on the chemical shifts of C-8/C-8′ and C-9/C-9′27, it was possible to infer that methyl groups at C-8 and C-8′ are positioned at trans configuration (Fig. 1).
Figure 1.
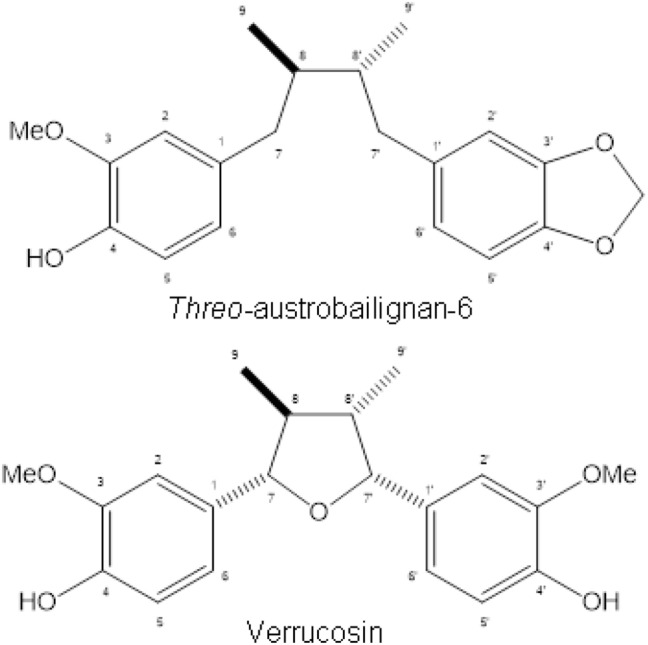
Chemical structures of neolignans isolated from S. cernuus.
NMR spectral data of verrucosin were similar to those reported in the literature28. Analysis of its 1H NMR spectrum, which showed a multiplet at δ 6.97—6.76 (H-2, H-5, H-6, H-2′, H-5′ and H-6′), two doublets of oxymethine hydrogens at δ 5.03 (J = 8.7, H-7) and 4.33 (J = 9.3, H-7′) and two doublets of methyl groups at δ 0.98 (J = 6.5 Hz, H-9) and 0.59 (J = 7.0 Hz, H-9′), indicated the occurrence of a tetrahydrofuran neolignan29. Additional singlets at δ 5.54 (4-OH), 5.49 (4′-OH), and 3.84 (3- and 3′-OMe) inferred the presence of two ortho methoxy-phenol moieties. 13C NMR spectrum showed signals referring to aromatic carbons from δ 109.4 to 146.5 (C-1 to C-6 and C-1′ to C-6′), two oxymethine carbons at δ 87.3 (C-7) and 83.1 (C-7′), two methine carbons at δ 47.8 (C-8) and 46.0 (C-8′), and two methyl groups at δ 14.5 (C-9) and 14.9 (C-9′). Furthermore, one intense signal at δ 55.9 was attributed to methoxyl groups at C-3 and C-3′. Based on the chemical shifts of C-7/C-7′, C-8/C-8´and C-9/C-9′30, it was possible to infer the stereochemistry trans,trans,cis to the substituents in the tetrahydrofuran groups (Fig. 1).
Finally, molecular formulas of isolated compounds were confirmed as C20H24O4 and C20H24O5 based on ESI-HRMS spectral data—threo-austrobailignan-6 shown [M—H]− ion peak at m/z 327.1594 whereas verrucosin exhibited [M + H]+ and [M + Na]+ ion peaks at m/z 345.1798 and 367.1518, respectively.
Threo-austrobailignan-6 and verrucosin affected the viability on adult S. mansoni
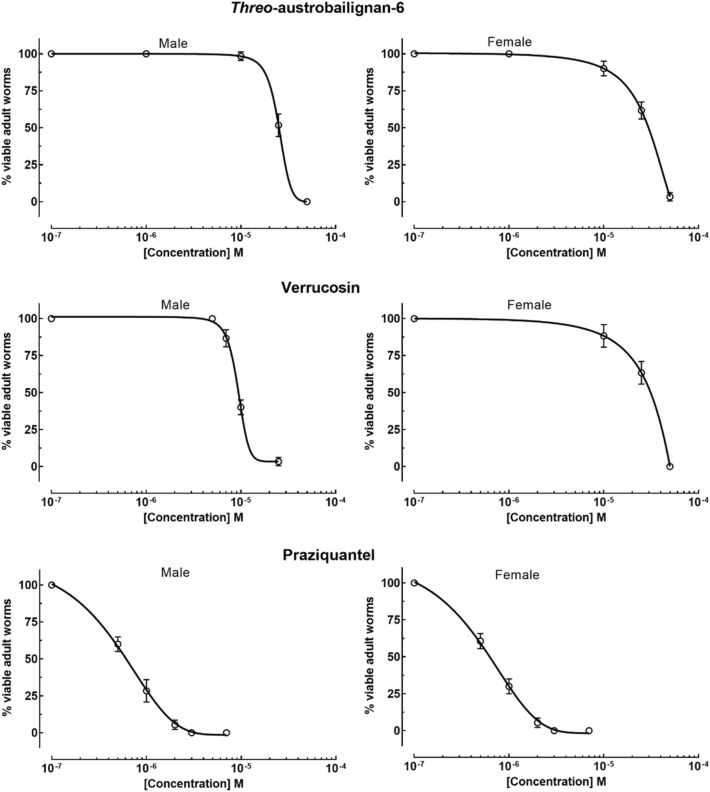
As reviewed elsewhere31, pharmacological studies demonstrated that plants of the genus Saururus have several compounds with biological activities, including anti-inflammatory and antitumor properties as well as antiprotozoal activities20,21. However, to the best of our knowledge, the effect of extracts or compounds from Saururus against human parasitic worms has not been described so far. In this study, to determine the antischistosomal potential of threo-austrobailignan-6 and verrucosin, S. mansoni worm pairs (male and female) were exposed to different concentrations for their effective concentration 50% (EC50) determination (Fig. 2). The known antiparasitic drug praziquantel was used as a reference. There were no modifications in the viability of the adult schistosomes belonging to the negative control groups for 72 h. As shown in Table 1, both verrucosin and threo-austrobailignan-6 presented schistosomicidal activity with EC50 values below 30 μM. Comparatively, verrucosin exhibited superior antiparasitic efficacy than threo-austrobailignan-6, with EC50 values ranging from 12.6 to 18.7 μM and 22.9 to 28.1 μM, respectively. The reference drug praziquantel was confirmed to be highly active, with an EC50 below 0.93 μM.
Figure 2.
Concentration–response curves for threo-austrobailignan-6, verrucosin, and praziquantel. Viability of ex vivo adult S. mansoni worms (male and female) was recorded within 72 h. Data are presented as the mean ± SD from a minimum of three experiments (n = 3). EC50 values are shown in Table 1.
Table 1.
In vitro activities of threo-austrobailignan-6, verrucosin, and praziquantel against S. mansoni adult worms and cytotoxicity.
| Compounds | S. mansoni EC50 (μM) | Monkey cells | Human cells | |||
|---|---|---|---|---|---|---|
| CC50 (μM) | SI | CC50 (μM) | SI | |||
| Male | Female | |||||
| Threo-austrobailignan-6 | 22.9 [18.2–28.6]* | 28.1 [23.6–34.7]* | > 500 | > 17.8 | > 500 | > 17.8 |
| Verrucosin | 12.6 [10.8–18.3]* | 18.7 [15.2–22.1]* | > 500 | > 26.8 | > 500 | > 26.8 |
| Praziquantel | 0.7 [0.6–0.8] | 0.8 [0.7–1.0] | > 500 | > 714 | > 500 | > 714 |
EC50, Effective concentration 50% against adult schistosomes; CC50, Cytotoxic concentration 50% against monkey (Vero) and human (HaCaT) cells; SI, Selectivity Index; *95% Confidence interval. The parasites were exposed for 72 h to the tested compounds to calculate the EC50. Values are calculated from three experiments, and each experiment was performed with three replicates. ND, not determined.
Based on the results obtained, tetrahydrofuran lignans, mainly verrucosin, have a potent schistosomicidal activity, and these compounds are more effective against schistosomes than other plant-derived products. For example, the following in vitro effective concentrations against S. mansoni adult worms were observed in the literature: 30 µM with neolignan licarin A25, 52 µM with monoterpene 3,7-dimethylloctanol32, 56.8 µM with monoterpene carvacrol33, 42.16 µM with carvacryl acetate, a derivative of carvacrol34, 85 µM with sesquiterpene nerolidol35, 50 µM with sesquiterpene cnicin36, 26.1 µM with diterpene ent-kaur-16-en-19-oic acid24, 50 µM with licochalcone A37, 50 µM 2-oxopopulifolic acid methyl ester, and 2-oxopopulifolic acid38, and 25 µM with chalcone cardamonin39.
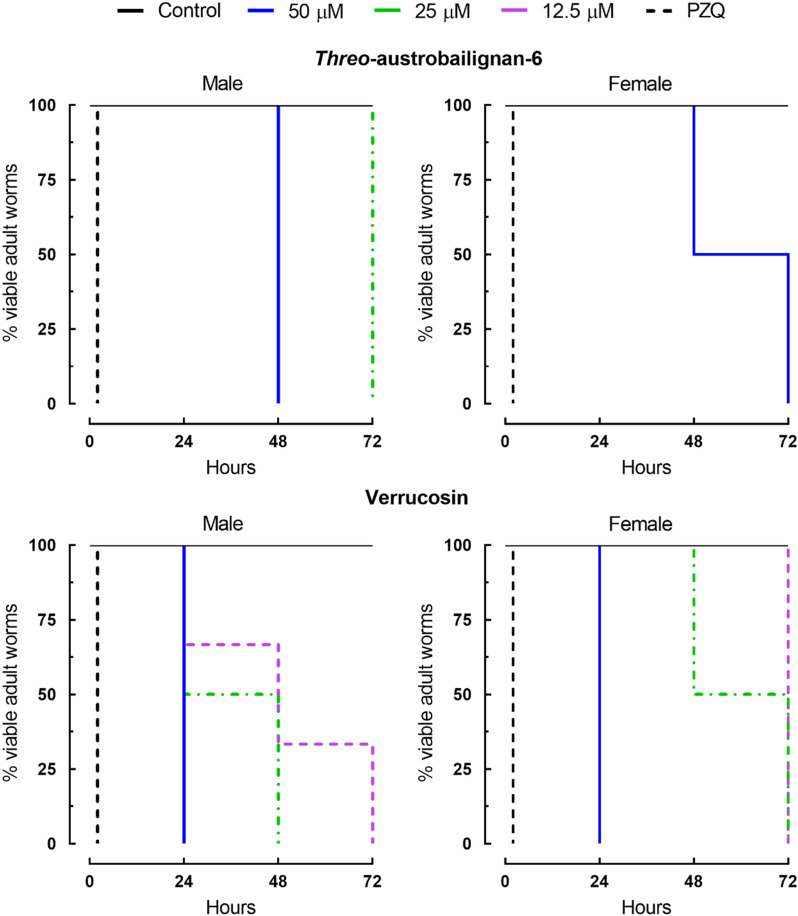
Assays regarding the survival times of adult worm pairs were also performed with tetrahydrofuran lignans, to understand the kinetics and mode of action of these substances. As shown in Fig. 3, these natural compounds induced mortality in a time- and concentration-dependent manner. Control parasites remained viable over the entire observation period, whereas praziquantel caused mortality of all worms immediately. The antischistosomal assay also revealed that verrucosin and threo-austrobailignan-6 were slightly more active against male S. mansoni. For example, verrucosin at 25 μM was lethal to 100% of male parasites after 48 h, whereas the death of all female worms was recorded within 72 h. When adult worm pairs were exposed to threo-austrobailignan-6 at a concentration of 25 μM for 72 h, 100% mortality was observed only for male worms. Similar to the anthelmintic properties of two tested neolignans, several studies have shown that male schistosomes are often more susceptible than female helminths40–42, and these results point to a sex-specific difference of tested compounds with the targets, but the mechanism of action remains to be elucidated. It should be noted that some compounds showed higher selectivity to female helminths, such as diterpene phytol43, or are equally active against both parasite sexes such as the prenylated benzophenone garcinielliptone FC44. It is known that male schistosomes had a higher sensitivity to praziquantel than female parasites45, corroborating the results obtained in this study. Multiple mechanisms are likely to be responsible for the difference in sensitivity between males and females exposed to verrucosin and threo-austrobailignan-6. Some of these may be due, in part, to the greater exposure of male worms to the surrounding environment. Indeed, because the S. mansoni female resides in the gynecophore canal, it may be less susceptible to attacks by anthelmintic molecules.
Figure 3.
Viability of ex vivo adult S. mansoni worms following exposure to neolignans. Adult worm pairs were obtained from mice by perfusion 42 days after infection. Parasites were monitored for up to 72 h, and results are expressed as the percent mortality recorded by Kaplan–Meier survival curves. Mean values of viability were derived from a minimum of three experiments (n = 3). Control: drug-free medium. PZQ: praziquantel at 2 µM.
Threo-austrobailignan-6 and verrucosin reduced S. mansoni egg production
The ability of tested tetrahydrofuran lignans to affect S. mansoni egg production was measured and the number of eggs within 72 h is shown in Fig. 4. All schistosomes of the negative control group showed normal viability and they remained paired and active throughout the treatment. Incubation of adult helminth pairs with either threo-austrobailignan-6 or verrucosin at a concentration of 50 μM kept the male and female parasites separated, which prevented the mating process, and a complete lack of oviposition was observed. At a concentration of 25 μM the parasites remained coupled, but the total number of egg worms was significantly reduced when compared to control worms (P < 0.0001 and P < 0.001 for verrucosin or threo-austrobailignan-6, respectively). A significant reduction in oviposition was also recorded with verrucosin at 12.5 μM (P < 0.01).
Figure 4.
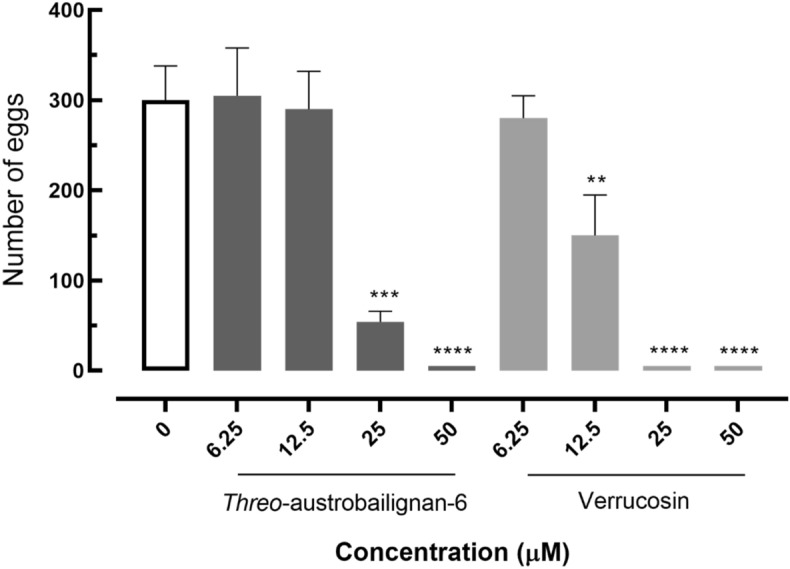
Eggs released by paired adult S. mansoni exposed to neolignans. Control: drug-free medium. Data are presented as the mean ± SD from three independent experiments (n = 3).
Considering that egg production is an essential mechanism for both transmission and pathogenesis, the negative impact on schistosomes egg-laying is of particular interest for molecules with anthelmintic activities. Indeed, the effects of antischistosomal agents on egg output during in vitro incubation have been previously reported in several studies46–49. Similar to these studies, threo-austrobailignan-6 and verrucosin, which also had a schistosomicidal effect, caused a reduction in S. mansoni egg production. The interference in oviposition may be associated with the separation of parasite couples or by changes in the reproductive system of S. mansoni42,49.
Threo-austrobailignan-6 and verrucosin caused morphological alterations to the schistosome tegument
Given the importance of the schistosomes’ tegument as a target for an anthelmintic agent50, we used scanning electron microscopy to examine the surface of S. mansoni adult parasite exposed to threo-austrobailignan-6 or verrucosin. The two neolignans tested at lethal concentrations caused morphological changes in the tegumental surfaces of male and female schistosomes. As shown in Fig. 5, adult parasites belonging to the control group (drug-free medium) showed an intact surface structure and topography (Fig. 5A,B). In contrast, helminths exposure to threo-austrobailignan-6 at 50 µM exhibited severe tegumental alterations such as swelling, sloughing, and shortening of the tubercles (Fig. 5C,D). Schistosomes incubated with verrucosin at 25 µM (Fig. 5E,F) and 50 µM (Fig. 5G,H) displayed substantial tegumental disruption throughout the whole body, with the tubercles losing their natural shape, and the spicules were markedly affected when compared to those of controls.
Figure 5.
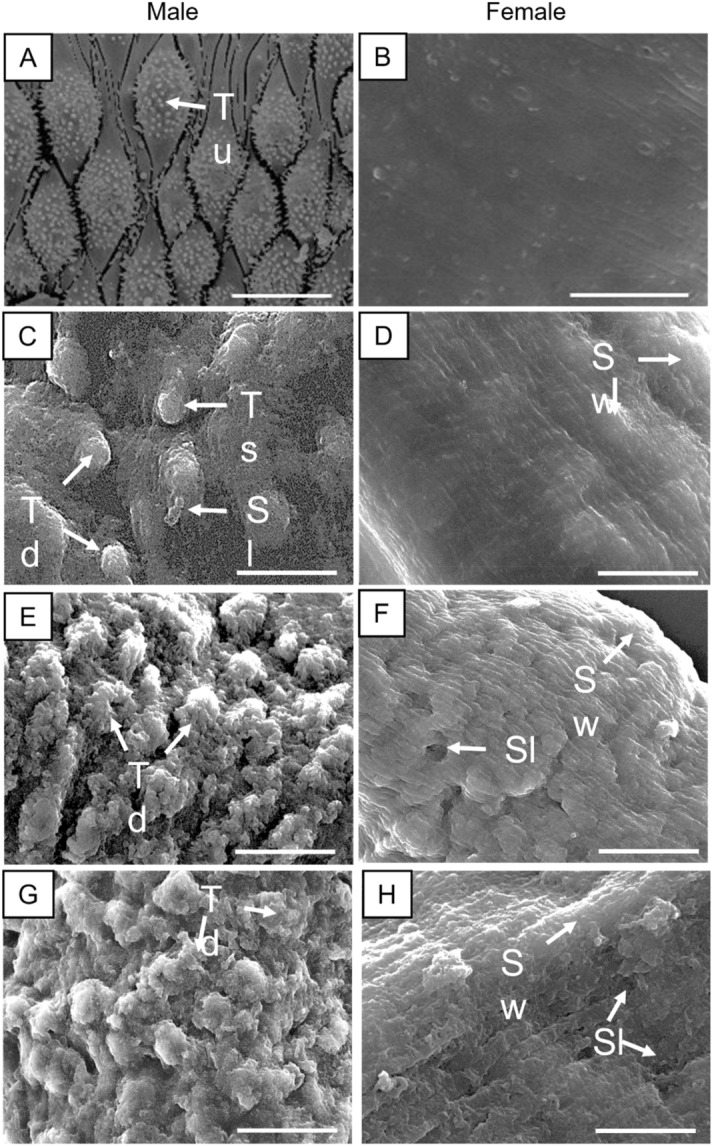
Scanning electron microscopy investigation of adult S. mansoni following incubation with neolignans. Control male worms showing intact tubercles (Tu) and spines on the surface (arrow) (A) and control female worms (B). Schistosomes were exposed to threo-austrobailignan-6 at 50 µM (C and D) or verrucosin at 25 µM (E and F) and 50 µM (G and H). Male (A, C, E, and G) and female (B, D, F, and H) helminths. The dorsal tegumental surface shows tubercle shortening (Ts), swelling (Sw), sloughing (Sl), and tubercle disintegration (Td). Images were captured using a JEOL SM 6460LV scanning electron microscope. Scale-bars: 10 µm.
Morphological alterations induced by several antischistosomal natural compounds on the tegument of S. mansoni have also been described. For example, a disintegration of the schistosomes’ surface was also observed after exposure to epiisopilosine48,51, piplartine23,52, and nerolidol40. Furthermore, it is known that praziquantel also causes morphological changes in the tegument of schistosomes42,53. Studies on anthelmintic activity of other lignans were described by de Paula Carlis and colleagues54, in a study which evaluated the effects of the hinokinin, cubebin, and dihydrocubebin isolated from Piper cubeta fruits, against eggs and third stage larvae of sheep gastrointestinal nematodes (Haemonchus contortus and Trichostrongylus sp). The authors reported that lignans caused serious lesions on the surface of parasites that led to death. Unlike nematodes, which are protected by a cuticle, schistosomes are covered by a living syncytium, called the tegument. Data from the present study clearly show that threo-austrobailignan-6 and verrucosin led to a pronounced change in the tegument of S. mansoni. Morphological alterations may not always result in the death of a parasite42,53,55, but the tegumental damage observed in this study could be a mechanism through which neolignans kill the schistosomes. From the point of view of an in vivo study, in addition to the direct effect on the survival of the schistosomes, tegumental alterations might result in exposure of the worm antigens to the host’s immune system56. Furthermore, considering that differential appearance of morphological changes was noted, it will be interesting to see the effect when the two drugs are combined in future experiments.
Taken together, this finding indicates that both threo-austrobailignan-6 and verrucosin have a significant antischistosomal effect and the morphological alterations could be a mechanism through which these neolignans kill the helminths. However, the exact mechanism is unclear and needs further investigation. In T. cruzi, Brito and coworkers21 reported that dibenzylbutane neolignans act on the plasma membrane of the parasite and induce alterations in the mitochondrial membrane potential. Moreover, because the redox system is responsible for the survival of many parasites, the authors demonstrated that neolignans cause alterations in the ROS production. Studies have reported that some antischistosomal compounds, such as licochalcone A, elicit mitochondrial and cellular membrane alterations in S. mansoni57. In addition, a wide range of natural compounds are known to generate ROS in S. mansoni, and the induction of oxidative stress has been considered an attractive treatment strategy for schistosomiasis drug discovery57–59. Accordingly, the possibility of threo-austrobailignan-6 and verrucosin causing disturbance in both ROS and the activities of enzymes associated with maintaining redox balance cannot be excluded.
Threo-austrobailignan-6 and verrucosin did not exhibit cytotoxicity
Isolated neolignans were incubated with two mammalian cell lines, namely the African green monkey kidney epithelial cells (Vero) and human epithelial cells (HaCaT) to evaluate their in vitro cytotoxic concentration 50% (CC50). The selectivity index (SI) was calculated as the ratio between the CC50 values for the human cells and the EC50 values for S. mansoni. As shown in Table 1, the tested compounds were non-cytotoxic to both animal and human cell lines. Accordingly, high SI values were determined, with SI > 17.8 and > 26.8 for threo-austrobailignan-6 and verrucosin, respectively. In line with this study, the low cytotoxic profile of neolignans from S. cernuus had been reported21. The SI of a compound is a widely accepted criterion used to express a drug’s in vitro efficacy60,61. Since the WHO establishes a SI value ≥ 10 for the selection of candidate anthelminthic compounds49,62, this study demonstrated that threo-austrobailignan-6 and verrucosin are highly selective antischistosomal agents.
In conclusion, neolignans threo-austrobailignan-6 and verrucosin, isolated from the freshwater plant S. cernuus, exhibited promising antiparasitic activity against blood fluke S. mansoni, inducing severe tegumental damage and a significant decrease in the production of eggs. In addition, these compounds displayed low cytotoxicity potential against both animal and human cell lines, resulting in relevant selectivity indices. Considering the promising chemical and biological properties of threo-austrobailignan-6 and verrucosin, these lignans could be used as starting points to develop new antischistosomal agents.
Methods
General procedures
1H and 13C NMR spectra were recorded on an Ultrashield 300 Bruker Avance III spectrometer (Billerica, MA, USA), operating, respectively, at 300 and 75 MHz. As a solvent and internal standard were used CDCl3 (Aldrich) and TMS (Aldrich). Chemical shifts are reported in δ units (ppm) and coupling constants (J) in Hz. ESI-HRMS spectra were measured on a Bruker Daltonics MicroTOF QII spectrometer. Silica gel 60 (Merck, 63–210 mesh) and Sephadex LH-20 (GE) were used for column chromatographic separation procedures whereas silica gel 60 PF254 (Merck) was used for analytical (0.25 mm) and preparative (1.00 mm) thin-layer chromatography (TLC).
Plant material
The use of plant parts in the present study complies with the international, national, and institutional guidelines. S. cernuus leaves were obtained from a local producer of ornamental plants in the city of Suzano, São Paulo State, Brazil in August/2019 and, after collection, received a registration code A4123E4 at SISGEN (National System for the Management of Genetic Heritage and Associated Traditional Knowledge—Ministry of the Environment, Brazil). Botanical identification was performed by Dr. Fátima Otavina de Souza Buturi, from University of São Judas Tadeu, and Dr. Oriana Aparecida Fávero, from Mackenzie Presbyterian University. A voucher specimen (voucher number E. A. Ferreira—001) has been deposited at the SPF Herbarium of the Institute of Biosciences at the University of São Paulo.
Isolation of threo-austrobailignan-6 and verrucosin
Dried leaves of S. cernuus (315 g) were powdered and exhaustively extracted using MeOH at room temperature to afford 91 g of crude extract after solvent evaporation at reduced pressure. This extract was resuspended in MeOH:H2O 1:1 and partitioned using hexane to afford 18.6 g of hexane phase. Part of this material (9.0 g) was subjected to column chromatography over silica gel eluted with n-hexane containing increasing amounts of EtOAc to afford fourteen fractions (A–N). After an initial screening, fractions C and G displayed activity against S. mansoni and were selected for further purification procedures. Therefore, fraction C (671 mg) was subjected to column chromatography over silica gel eluted with n-hexane containing increasing amounts of EtOAc to afford six fractions (C1–C6). Fraction C3 (232 mg) was chromatographed over a silica gel column eluted with n-hexane:EtOAc 3:2 to afford the fraction C3-4 (25 mg) which was purified by prep. TLC (20 × 20 cm2, toluene:Me2CO 7:3). This procedure gave threo-austrobailignan-6 (13 mg)21. Part of fraction G (760 mg) was chromatographed over Sephadex LH-20 eluted with n-hexane:CH2Cl2 1:4 and CH2Cl2:acetone 3:2 to afford pure verrucosin (335 mg).
NMR and ESI-HRMS data of threo-austrobailignan-6 and verrucosin
Threo-austrobailignan-6. White amorphous solid. 1H NMR (CDCl3), δ /ppm 6.80 (d, J = 7.9 Hz, H-5′), 6.70 (d, J = 7.8 Hz, H-5), 6.67 (d, J = 1.7 Hz, H-2), 6.60 (d, J = 1.7 Hz, H-2′), 6.57–6.53 (m, H-6/H-6′), 5.93 (s, OCH2O), 3.85 (s, OCH3), 2.57 (dd, J = 13.5 and 6.5 Hz, H-7b), 2.56 (dd, J = 13.5 and 6.5 Hz, H-7′b), 2.39 (dd, J = 13.5 and 8.0 Hz, H-7a), 2.33 (dd, J = 13.5 and 8.0 Hz, H-7′a), 1.76–1.67 (m, H-8/H-8′), 0.86 (d, J = 5.9 Hz, H-9), 0.81 (d, J = 6.6 Hz, H-9′). 13C NMR (CDCl3), δ /ppm 147.3 (C-3), 146.3 (C-3′), 145.4 (C-4), 143.5 (C-4′), 135.5 (C-1), 133.5 (C-1′), 121.8 (C-6), 121.6 (C-6′), 114.0 (C-5′), 111.3 (C-2′), 109.3 (C-2), 107.9 (C-5), 100.7 (OCH2O), 55.8 (OCH3), 41.1 (C-7), 41.0 (C-7′), 37.9 (C-8), 37.8 (C-8′), 13.9 (C-9), 13.8 (C-9′). ESI-HRMS m/z 327.1594 [M—H]− (calcd for C20H23O4 327.1596).
Verrucosin. White amorphous solid. 1H NMR (CDCl3), δ /ppm 6.97–6.76 (m, H-2/H-2′, H-5/H-5′, and H-6/H-6′), 5.03 (d, J = 8.7 Hz, H-7), 5.54 (4-OH), 5.49 (4′-OH),4.33 (d, J = 9.3 Hz, H-7′), 3.84 (s, 3-OMe), 3.78 (s, 3′-OMe), 2.20–2.11 (m, H-8), 1.75–1.64 (m, H-8′), 0.98 (d, J = 6.5 Hz, H-9), 0.59 (d, J = 7.0 Hz, H-9′). 13C NMR (CDCl3), δ /ppm 146.5 (C-3), 146.2 (C-3′), 145.2 (C-4), 144.6 (C-4′), 133.2 (C-1), 132.8 (C-1′), 119.9 (C-6′), 119.3 (C-6), 114.2 (C-5), 113.9 (C-5′), 109.7 (C-2′), 109.4 (C-2), 87.3 (C-7), 83.1 (C-7′), 55.9 (3-/3′-OMe), 47.8 (C-8), 46.0 (C-8′), 14.9 (C-9′), 14.5 (C-9). ESI-HRMS m/z 345.1798 [M + H]+ and 367.1518 [M + Na]+ (calcd for C20H25O5 345.1702 and for C20H24O5Na 367.1521).
All the structural data collected are presented in the Supplementary Information (Fig. S1-S6).
Drugs and reagents
Dulbecco’s Modified Eagle Medium (DMEM) modified to contain 4 mM of L-glutamine, 4500 mg/L glucose, and 1 mM sodium pyruvate, Roswell Park Memorial Institute (RPMI) 1640 medium, trypsin/EDTA solutions, heat-inactivated fetal calf serum, and penicillin G-streptomycin solutions (10,000 U/ml penicillin G sodium salt, 10 mg/ml streptomycin sulfate) were obtained from Vitrocell (Campinas, SP, Brazil). HEPES buffer, thiazolyl blue tetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO, USA). Praziquantel was kindly provided by Ecovet Indústria Veterinária Ltda (São Paulo, SP, Brazil). In all in vitro experiments, compounds were solubilized in DMSO.
Animals, parasites, and cell lines
The life cycle of S. mansoni (BH strain) is maintained by routine passage through Biomphalaria glabrata snails and Swiss mice at Guarulhos University (UNG, Guarulhos, SP, Brazil). Rodents, three weeks-old, were purchased from Animais de Laboratório Criação e Comércio (Paulínea, SP, Brazil). Both mice and snails were kept under environmentally controlled conditions (25 °C; humidity of 50%), with free access to food and water. Cercariae of S. mansoni were obtained from infected intermediate host snails in our laboratories as described previously63,64.
HaCaT (human epithelial cells) and Vero (monkey kidney cells) were obtained from the Banco de Células do Rio de Janeiro (BCRJ, RJ, Brazil) and the American Type Culture Collection (ATCC CCL-81; Manassas, VA, USA), respectively. Cells were cultured in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a humidified atmosphere containing 5% CO2. They were maintained in 25 cm2 culture flasks (Corning, Tewksbury, MA, USA) and harvested using 0.25% trypsin in 0.2 g/L EDTA solution.
Antiparasitic assay
The antischistosomal assay was performed as previously described65,66. Briefly, S. mansoni adult parasites were obtained from infected mice at day 42 post-infection (parasite ex vivo). One adult male and one adult female worms (i.e. one worm pairs) were incubated in flat bottom 24-well plates (Corning, New York, NY, USA) in the presence of threo-austrobailignan-6 and verrucosin (started at 50 µM and followed a twofold dilution series) in RPMI 1640 culture medium supplemented with 5% inactivated fetal calf serum and containing antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). The control schistosomes were assayed in a culture medium and 0.5% DMSO (representing the highest concentration of solvent). Each concentration (50, 25, 12.5, 6.25, 3.12 µM) was tested in five replicates, and the experiments were repeated three times. Parasites were kept for 72 h (37 °C, 5% CO2), and their viability was monitored microscopically at 2, 24, 48, and 72 h using both a Motic AE2000 inverted microscope (Vancouver, Canada) and a Leica EZ4E stereomicroscope (Wetzlar, Germany). The death of adult schistosomes was defined as no movement observed for at least 1 to 2 min of examination, whereas parasites with any body movement were considered viable67. The percentage of viable adult schistosomes was calculated considering worms exposed to compounds vs control worms. For assessment of the reproductive fitness of S. mansoni, the number of eggs was counted daily using an inverted microscope (Motic) as previously described68.
Scanning electron microscopy investigation
Scanning electron microscopy studies were performed as previously described69,70. Briefly, adult worms (treated and control groups) were fixed in 2.5% glutaraldehyde, and mounted specimens were coated with gold sputter (Denton Vacuum LLC, Moorestown, NJ, USA) and photographed using a high-resolution scanning electron microscope (Jeol-JSM-6460LV, Tokyo, Japan).
Cytotoxicity assay
The MTT assay was used to evaluate the cytotoxic activity as previously described71,72. Briefly, cells were seeded (2 × 103/well in 96-well culture plates) and incubated with threo-austrobailignan-6 and verrucosin (started at 500 µM and followed a threefold dilution series) for 72 h at 37 °C and 5% CO2. MTT solution was added to each well and the absorbance was read on an Epoch Microplate Spectrophotometer (BioTek Instruments, Winooski, VT, USA) at 595 nm. The selectivity indices (SI) were calculated by dividing the 50% cytotoxic concentration (CC50) obtained on cells with 50% effective concentration (EC50) values determined on schistosomes73.
Statistical analysis
Statistical analyses were performed using Graph Pad Prism software 8.0 (San Diego, CA, USA). Data are presented as the mean ± standard deviation (SD) of at least three independent experiments, EC50 and, CC50 values were calculated using sigmoid dose–response curves74. For S. mansoni egg production, significant differences were determined by one-way analysis of variance (ANOVA) and applying Tukey's test for multiple comparisons with a level of significance set at P < 0.05.
Ethical approval
Animal studies are reported in compliance with the ARRIVE guidelines. The protocol for experimental design was reviewed and approved by the Committee for the Ethical Use of Animals in Experimentation of the Guarulhos University (Guarulhos, SP, Brazil; protocol ID 47/20) in conformity with the Brazilian law for Guidelines for Care and Use of Laboratory Animals.
Supplementary Information
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants 2020/01441-4 to JdeM and 2021/02789-7 to JHGL). JRB, DBR, MMG, and DCSS thanks for fellowships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). JdeM and JHGL also received an established investigator fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The funding institutions did not have any role in study design, data collection, data analysis, interpretation or writing of the report in this study. We thank Ana C. Mengarda for support in maintenance of the S. mansoni life cycle. We are also grateful to Mariana B.A. Silva for technical assistance.
Author contributions
J.R.B., J.H.G.L., P.W., and J.d.M. contributed to conceptualization; J.R.B., D.B.R., B.C.P., D.C.S.S., M.M.G., E.A.F., M.C.S., and F.S.T. contributed to experiments and data interpretation; J.R.B., D.B.R., B.C.P., D.C.S.S., E.A.F., M.C.S., F.S.T., and P.W. contributed to writing—original draft and visualization; J.H.G.L. and J.d.M. contributed with resources, writing—review & editing, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data availability
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Juliana R. Brito and Polrat Wilairatana.
Contributor Information
Polrat Wilairatana, Email: polrat.wil@mahidol.ac.th.
João Henrique G. Lago, Email: joao.lago@ufabc.edu.br
Josué de Moraes, Email: moraesnpdn@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-23110-2.
References
- 1.World Health Organization. Schistosomiasis. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (2022).
- 2.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat. Rev. Dis. Primers. 2018;4:30093684. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 3.Lago EM, Xavier RP, Teixeira TR, Silva LM, da Silva Filho AA, de Moraes J. Antischistosomal agents: State of art and perspectives. Future Med. Chem. 2018;10:89–120. doi: 10.4155/fmc-2017-0112. [DOI] [PubMed] [Google Scholar]
- 4.Mengarda AC, Iles B, Longo JPF, de Moraes J. Recent trends in praziquantel nanoformulations for helminthiasis treatment. Expert. Opin. Drug Deliv. 2022;14:1–11. doi: 10.1080/17425247.2022.2051477. [DOI] [PubMed] [Google Scholar]
- 5.Deol AK, et al. Schistosomiasis—Assessing progress toward the 2020 and 2025 global goals. N. Engl. J. Med. 2019;381:2519–2528. doi: 10.1056/NEJMoa1812165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assaré RK, et al. Characteristics of persistent hotspots of Schistosoma mansoni in western Côte d'Ivoire. Parasit. Vectors. 2020;13:337. doi: 10.1186/s13071-020-04188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg RM. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology. 2013;140:1534–1546. doi: 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabuyaya M, Chimbari MJ, Mukaratirwa S. Efficacy of praziquantel treatment regimens in pre-school and school aged children infected with schistosomiasis in sub-Saharan Africa: A systematic review. Infect. Dis. Poverty. 2018;7:1–7. doi: 10.1186/s40249-018-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis—A meta-analysis of comparative and noncomparative clinical trials. PLoS Negl. Trop. Dis. 2014;8:e3286. doi: 10.1371/journal.pntd.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwang J, Olliaro P. Efficacy and safety of praziquantel 40 mg/kg in preschool-aged and school-aged children: A meta-analysis. Parasit. Vectors. 2017;10:47. doi: 10.1186/s13071-016-1958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Ending the neglect to attain the sustainable development goals: A road map for neglected tropical diseases 2021–2030. https://www.who.int/publications/i/item/9789240010352 (2021).
- 12.Mafud AC, Ferreira LG, Mascarenhas YP, Andricopulo AD, de Moraes J. Discovery of novel antischistosomal agents by molecular modeling approaches. Trends Parasitol. 2016;32:874–886. doi: 10.1016/j.pt.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 13.de Moraes J, Geary TG. FDA-approved antiparasitic drugs in the 21st century: A success for helminthiasis? Trends Parasitol. 2020;36:573–575. doi: 10.1016/j.pt.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira LLG, de Moraes J, Andricopulo AD. Approaches to advance drug discovery for neglected tropical diseases. Drug Discov. Today. 2022;27:2278–2287. doi: 10.1016/j.drudis.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Kubanek J, Fenical W, Hay ME, Brown PJ, Lindquist N. Two antifeedant lignans from the freshwater macrophyte Saururus cernuus. Phytochemistry. 2000;54:281–287. doi: 10.1016/s0031-9422(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 16.Hodges TW, Hossain CF, Kim YP, Zhou YD, Nagle DGJ. Molecular-targeted antitumor agents: The Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of hypoxia-activated HIF-1. J. Nat. Prod. 2004;67:767–771. doi: 10.1021/np030514m. [DOI] [PubMed] [Google Scholar]
- 17.Rao KV, Rao NSP. Chemistry of Saururus cernuus, VI: three new neolignans. J. Nat. Prod. 1990;53:212–215. doi: 10.1021/np50067a036. [DOI] [PubMed] [Google Scholar]
- 18.Hossain CF, et al. Saururus cernuus lignans–potent small molecule inhibitors of hypoxia-inducible factor-1. Biochem. Biophys. Res. Commun. 2005;333:1026–1033. doi: 10.1016/j.bbrc.2005.05.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao T, Li F, Huang L, Liang J, Qu W. Secondary metabolites from the plants of the family Saururaceae and their biological properties. Chem. Biodivers. 2015;12:194–220. doi: 10.1002/cbdv.201300342. [DOI] [PubMed] [Google Scholar]
- 20.Brito JR, et al. Antileishmanial activity and ultrastructural changes of related tetrahydrofuran dineolignans isolated from Saururus cernuus L. (Saururaceae) J. Pharm. Pharmacol. 2019;71:1871–1878. doi: 10.1111/jphp.13171. [DOI] [PubMed] [Google Scholar]
- 21.Brito JR, Costa-Silva TA, Tempone AG, Ferreira EA, Lago JHG. Dibenzylbutane neolignans from Saururus cernuus L. (Saururaceae) displayed anti-Trypanosoma cruzi activity via alterations in the mitochondrial membrane potential. Fitoterapia. 2019;137:104251. doi: 10.1016/j.fitote.2019.104251. [DOI] [PubMed] [Google Scholar]
- 22.Brito JR, et al. Molecular dereplication of volatile oils from Saururus cernuus L. and evaluation of anti-Trypanosoma cruzi activity. Quim. Nova. 2022;45:159–164. doi: 10.21577/0100-4042.20170811. [DOI] [Google Scholar]
- 23.Mengarda AC, et al. Antiparasitic activity of piplartine (piperlongumine) in a mouse model of schistosomiasis. Acta Trop. 2020;205:105350. doi: 10.1016/j.actatropica.2020.105350. [DOI] [PubMed] [Google Scholar]
- 24.Sessa DP, Mengarda AC, Simplicio PE, Antar GM, Lago JHG, de Moraes J. 15β-senecioyl-oxy-ent-kaur-16-en-19-oic acid, a diterpene isolated from Baccharis lateralis, as promising oral compound for the treatment of schistosomiasis. J. Nat. Prod. 2020;83:3744–3750. doi: 10.1021/acs.jnatprod.0c01050. [DOI] [PubMed] [Google Scholar]
- 25.Mengarda AC, et al. Licarin A, a neolignan isolated from Nectandra oppositifolia Nees & Mart. (Lauraceae), exhibited moderate preclinical efficacy against Schistosoma mansoni infection. Phytother. Res. 2021;35:5154–5162. doi: 10.1002/ptr.7184. [DOI] [PubMed] [Google Scholar]
- 26.Hattori M, Hada S, Kawata Y, Tezuka Y, Kikuchi T, Namba T. New 2,5-bis-aryl 3,4-dimethyltetrahydrofuran lignans from the aril of Myristica fragrans. Chem. Pharm. Bull. 1987;35:3315–3322. doi: 10.1248/cpb.35.3315. [DOI] [Google Scholar]
- 27.Herath HMTB, Priydarshini AMA. Lignans from Myristica dactyloides. Phytochemistry. 1997;44:699–703. doi: 10.1016/S0031-9422(96)00607-3. [DOI] [Google Scholar]
- 28.Vasquez JDG, Suárez LEC, Martínez JHI. Fragmentación diastereoespecífica en nci de dos lignanos dibencilbutánicos de la corteza de Dugandiodendron argyrotrichum (Magnoliaceae) Rev. Prod. Nat. 2008;2:6–12. doi: 10.3407/rpn.v2i1.17. [DOI] [Google Scholar]
- 29.Ponci V, et al. Neolignans from Nectandra megapotamica (Lauraceae) display in vitro cytotoxic activity and induce apoptosis in leukemia cells. Molecules. 2015;20:12757–12768. doi: 10.3390/molecules200712757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui H, Xu B, Wu T, Xu J, Yuan Y, Gu Q. Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-Barr virus lytic replication. J. Nat. Prod. 2014;77:100–110. doi: 10.1021/np400757k. [DOI] [PubMed] [Google Scholar]
- 31.Liu G, et al. A review of traditional uses, phytochemistry, and pharmacological properties of the genus Saururus. Am. J. Chin. Med. 2020;48:47–76. doi: 10.1142/S0192415X20500032. [DOI] [PubMed] [Google Scholar]
- 32.Mafud AC, et al. Structural parameters, molecular properties, and biological evaluation of some terpenes targeting Schistosoma mansoni parasite. Chem. Biol. Interact. 2016;244:129–139. doi: 10.1016/j.cbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Xavier ES, et al. Therapeutic efficacy of carvacrol-loaded nanoemulsion in a mouse model of schistosomiasis. Front Pharmacol. 2022;13:917363. doi: 10.3389/fphar.2022.917363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva BC, et al. Efficacy of carvacryl acetate in vitro and following oral administration to mice harboring either prepatent or patent Schistosoma mansoni infections. Parasitol. Res. 2021;120:3837–3844. doi: 10.1007/s00436-021-07333-2. [DOI] [PubMed] [Google Scholar]
- 35.Silva MP, et al. Antiparasitic activity of nerolidol in a mouse model of schistosomiasis. Int. J. Antimicrob. Agents. 2017;50:467–472. doi: 10.1016/j.ijantimicag.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Queiroz LS, et al. In vitro and in vivo evaluation of cnicin from blessed thistle (Centaurea benedicta) and its inclusion complexes with cyclodextrins against Schistosoma mansoni. Parasitol. Res. 2021;120:1321–1333. doi: 10.1007/s00436-020-06963-2. [DOI] [PubMed] [Google Scholar]
- 37.Silva LM, et al. Licochalcone A-loaded solid lipid nanoparticles improve antischistosomal activity in vitro and in vivo. Nanomedicine. 2021;16:1641–1655. doi: 10.2217/nnm-2021-0146. [DOI] [PubMed] [Google Scholar]
- 38.Costa PDS, et al. Assessment of the in vitro antischistosomal activities of the extracts and compounds from Solidago microglossa DC (Asteraceae) and Aristolochia cymbifera Mart. & Zucc. (Aristolochiaceae) Evid. Based Complement. Alternat. Med. 2020;2020:1726365. doi: 10.1155/2020/1726365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Castro CC, et al. Cardamonin, a schistosomicidal chalcone from Piper aduncum L. (Piperaceae) that inhibits Schistosoma mansoni ATP diphosphohydrolase. Phytomedicine. 2015;22:921–928. doi: 10.1016/j.phymed.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Silva MP, et al. Antischistosomal activity of the terpene nerolidol. Molecules. 2014;19:3793–37803. doi: 10.3390/molecules19033793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Carvalho LSA, et al. In vitro schistosomicidal activity of the alkaloid-rich fraction from Ruta graveolens L. (Rutaceae) and its characterization by UPLC-QTOF-MS. Evid. Based Complement. Alternat. Med. 2019;2019:7909137. doi: 10.1155/2019/7909137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva MP, et al. Brazilian red propolis exhibits antiparasitic properties in vitro and reduces worm burden and egg production in an mouse model harboring either early or chronic Schistosoma mansoni infection. J. Ethnopharmacol. 2021;264:113387. doi: 10.1016/j.jep.2020.113387. [DOI] [PubMed] [Google Scholar]
- 43.de Moraes J, et al. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2018;8:e2617. doi: 10.1371/journal.pntd.0002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva AP, et al. Garcinielliptone FC: Antiparasitic activity without cytotoxicity to mammalian cells. Toxicol. In Vitro. 2015;29:681–687. doi: 10.1016/j.tiv.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Guimarães MA, et al. Anthelmintic activity in vivo of epiisopiloturine against juvenile and adult worms of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015;9:e0003656. doi: 10.1371/journal.pntd.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Almeida LM, et al. Flavonoids and sesquiterpene lactones from Artemisia absinthium and Tanacetum parthenium against Schistosoma mansoni worms. Evid. Based Complement. Alternat. Med. 2016;2016:9521349. doi: 10.1155/2016/9521349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha JA, et al. Anthelmintic, antibacterial and cytotoxicity activity of imidazole alkaloids from Pilocarpus microphyllus leaves. Phytother. Res. 2017;31:624–630. doi: 10.1002/ptr.5771. [DOI] [PubMed] [Google Scholar]
- 49.Morais CS, et al. Pyrazoline derivatives as promising novel antischistosomal agents. Sci. Rep. 2021;11:23437. doi: 10.1038/s41598-021-02792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moraes J. 2000 Antischistosomal natural compounds: present challenges for new drug screens. In: Rodriguez-Morales AJ, editor. Current Topics in Tropical Medicine. InTech; 2000. pp. 333–358. [Google Scholar]
- 51.Guimarães MA, et al. Epiisopilosine alkaloid has activity against Schistosoma mansoni in mice without acute toxicity. PLoS ONE. 2018;13:e0196667. doi: 10.1371/journal.pone.0196667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campelo YDM, et al. Synergistic effects of in vitro combinations of piplartine, epiisopiloturine and praziquantel against Schistosoma mansoni. Biomed. Pharmacother. 2017;88:488–499. doi: 10.1016/j.biopha.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 53.de Moraes J, et al. In vitro synergistic interaction between amide piplartine and antimicrobial peptide dermaseptin against Schistosoma mansoni schistosomula and adult worms. Curr. Med. Chem. 2013;20:301–309. doi: 10.2174/092986713804806694. [DOI] [PubMed] [Google Scholar]
- 54.de Paula Carlis MS, et al. In vitro anthelmintic activity of the crude hydroalcoholic extract of Piper cubeba fruits and isolated natural products against gastrointestinal nematodes in sheep. Vet. Parasitol. 2019;275:108932. doi: 10.1016/j.vetpar.2019.108932. [DOI] [PubMed] [Google Scholar]
- 55.de Moraes J, Dario BS, Couto RA, Pinto PL, da Costa Ferreira AM. Antischistosomal activity of oxindolimine-metal complexes. Antimicrob. Agents Chemother. 2015;59:6648–6652. doi: 10.1128/AAC.01371-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doenhoff MJ, Sabah AA, Fletcher C, Webbe G, Bain J. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Trans. R. Soc. Trop. Med. Hyg. 1987;81:947–951. doi: 10.1016/0035-9203(87)90360-9. [DOI] [PubMed] [Google Scholar]
- 57.Souza RL, et al. Licochalcone A induces morphological and biochemical alterations in Schistosoma mansoni adult worms. Biomed. Pharmacother. 2017;96:64–71. doi: 10.1016/j.biopha.2017.09.128. [DOI] [PubMed] [Google Scholar]
- 58.Sayed AA, Cook SK, Williams DL. Redox balance mechanisms in Schistosoma mansoni rely on peroxiredoxins and albumin and implicate peroxiredoxins as novel drug targets. J. Biol. Chem. 2006;281:17001–17010. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]
- 59.Badoco FR, et al. EF24, a schistosomicidal curcumin analog: Insights from its synthesis and phenotypic, biochemical and cytotoxic activities. Chem. Biol. Interact. 2022;2022:110191. doi: 10.1016/j.cbi.2022.110191. [DOI] [PubMed] [Google Scholar]
- 60.Katsuno K, et al. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015;14:751–758. doi: 10.1038/nrd4683. [DOI] [PubMed] [Google Scholar]
- 61.Tempone AG, et al. Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria. Nat. Prod. Rep. 2021;38:2214–2235. doi: 10.1039/d0np00078g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pink R, Hudson A, Mouriès MA, Bendig M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005;4:727–740. doi: 10.1038/nrd1824. [DOI] [PubMed] [Google Scholar]
- 63.Lago EM, et al. Phenotypic screening of nonsteroidal anti-inflammatory drugs identified mefenamic acid as a drug for the treatment of schistosomiasis. EBioMedicine. 2019;43:370–379. doi: 10.1016/j.ebiom.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Porto R, et al. Antiparasitic properties of cardiovascular agents against human intravascular parasite Schistosoma mansoni. Pharmaceuticals (Basel) 2021;14:686. doi: 10.3390/ph14070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roquini DB, et al. Promethazine exhibits antiparasitic properties in vitro and reduces worm burden, egg production, hepato-, and splenomegaly in a schistosomiasis animal model. Antimicrob. Agents Chemother. 2019;63:e01208–e1219. doi: 10.1128/AAC.01208-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xavier RP, et al. H1-antihistamines as antischistosomal drugs: In vitro and in vitro studies. Parasit. Vectors. 2020;13:278. doi: 10.1186/s13071-020-04140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silva TC, et al. New evidence for tamoxifen as an antischistosomal agent: In vitro, in vitro and target fishing studies. Future Med. Chem. 2021;13:945–957. doi: 10.4155/fmc-2020-0311. [DOI] [PubMed] [Google Scholar]
- 68.Mafud AC, et al. Antiparasitic, structural, pharmacokinetic, and toxicological properties of riparin derivatives. Toxicol. In Vitro. 2018;50:1–10. doi: 10.1016/j.tiv.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 69.de Brito MRM, et al. Cyclohexene-fused 1,3-oxazines with selective antibacterial and antiparasitic action and low cytotoxic effects. Toxicol. In Vitro. 2017;44:273–279. doi: 10.1016/j.tiv.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 70.Guerra RA, et al. In vitro and in vitro studies of spironolactone as an a antischistosomal drug capable of clinical repurposing. Antimicrob. Agents Chemother. 2019;63:e01722–e1818. doi: 10.1128/AAC.01722-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amorim CR, et al. Schiff bases of 4-phenyl-2-aminothiazoles as hits to new antischistosomal: Synthesis, in vitro, in vitro and in silico studies. Eur. J. Pharm. Sci. 2020;150:105371. doi: 10.1016/j.ejps.2020.105371. [DOI] [PubMed] [Google Scholar]
- 72.Silva TC, et al. N-(4-methoxyphenyl)pentanamide, a simplified derivative of albendazole, displays anthelmintic properties against the nematode Toxocara canis. Microbiol. Spectr. 2022;28:e0180722. doi: 10.1128/spectrum.01807-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Brito MG, et al. Therapeutic effect of diminazene aceturate on parasitic blood fluke Schistosoma mansoni infection. Antimicrob. Agents Chemother. 2020;64:e01372–e1420. doi: 10.1128/AAC.01372-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roquini DB, Silva GL, Ferreira LLG, Andricopulo AD, Wilairatana P, De Moraes J. Susceptibility of Angiostrongylus cantonensis larvae to anthelmintic drugs. Front. Pharmacol. 2022;13:901459. doi: 10.3389/fphar.2022.901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.