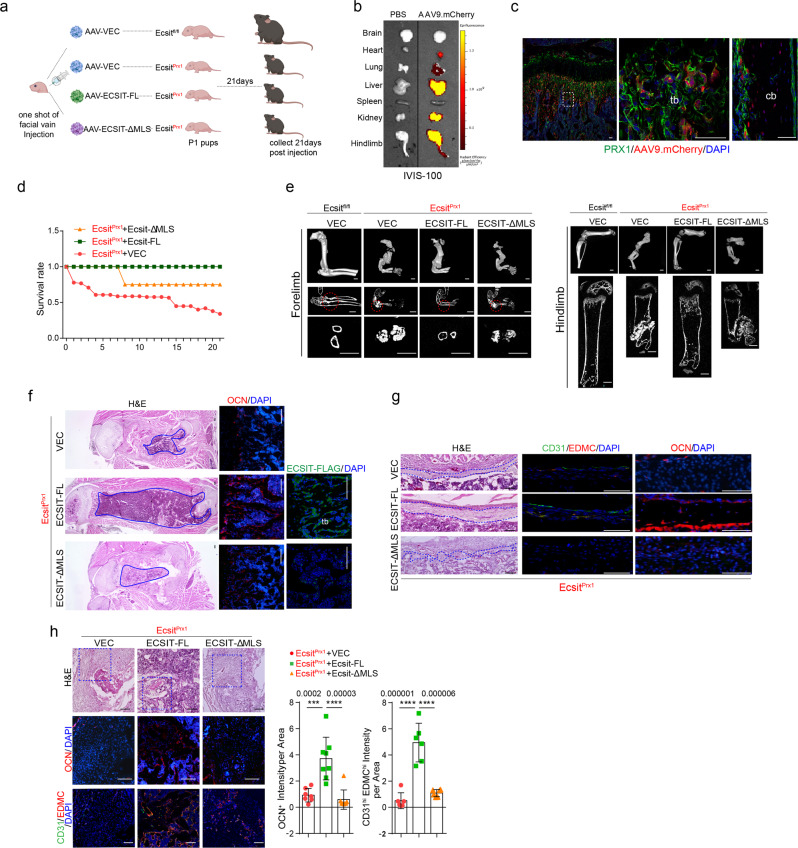
Fig. 3. AAV-mediated expression of ECSIT reverses EcsitPrx1 skeletal phenotypes.
a Diagram summarizing the study and treatment methods. A single dose of 2 x 1011 genome copies (GCs) of rAAV9 vectors carrying control vector or FLAG-ECSIT constructs was injected into P1 EcsitPrx1 neonates via the facial vein and musculoskeletal phenotypes were assessed 21 days post-injection (created with biorender.com). b Single dose of 2 x 1011 GCs of rAAV9 carrying mCherry was injected into P0 Prx1;Rosa26mTmG neonates via facial vein and mCherry expression was monitored by IVIS-100 optical imaging 21 days post-injection. c rAAV9 vector carrying mCherry was injected into Prx1;Rosa26mTmG neonates and 21 days later, mCherry expression was assessed by fluorescence microscopy of cryo-sectioned femurs. d Survival rate of AAV-treated EcsitPrx1 mice up to 21 days post-injection (n = 6). e MicroCT analysis of the forelimbs (left) and hindlimbs (right) of P21 AAV-treated Ecsitfl/fl and EcsitPrx1 mice (n = 5–8). f Longitudinal sections of P21 AAV-treated Ecsitfl/fl and EcsitPrx1 femurs were stained for H&E (left) or immunostained for OCN (middle) or FLAG-ECSIT (right). Blue lines indicate the bone marrow area. g H&E staining (left) or immunofluorescence for CD31, EDMC, or OCN (middle, right) of diaphyseal cortical bones of P21 AAV-treated Ecsitfl/fl and EcsitPrx1 femurs. Blue lines indicate periosteum. h H&E staining (top) or immunofluorescence for CD31, EDMC (VEC, n = 7; ECSIT-FL, n = 8, ECSIT-MLS, n = 6), or OCN (n = 6, middle, bottom) of fracture sites of P21 AAV-treated Ecsitfl/fl and EcsitPrx1 femurs. Immunofluorescence intensity was quantified using ImageJ software. An ordinary one-way ANOVA with Dunnett’s multiple comparisons test (h; error bars, data represent mean ± SD). Data are representative of three independent experiments. Scale bars = c, 50 μm; e, 1 mm; f, h, 100 μm; g, 75 μm.