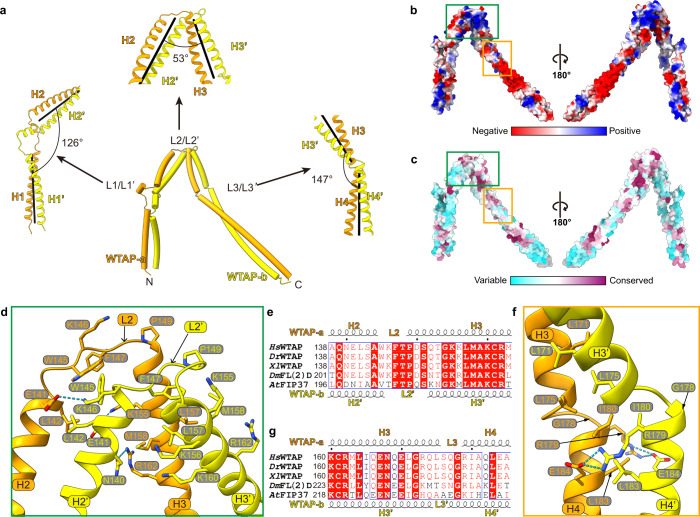
Fig. 3. WTAP forms a saddle-shaped homodimer through coiled-coil interaction.
a Overall structure of WTAP homodimer. The distortion angles of the axes of coiled-coils at L1, L2, and L3 are shown in the left, upper, and right panels, respectively. The axes of coiled-coils are shown as black lines in each panel. b, c Two different views of the WTAP homodimer surface colored by electrostatic potential (b) and sequence conservation (c). d Intermolecular contacts between WTAP-a and WTAP-b around L2/L2’, corresponding to the green box in b and c. e Sequence alignment of the WTAP homodimer (138–163) containing the L2/L2’ region. Hs, Homo sapiens; Dr, Danio rerio; Xl, Xenopus laevis; Dm, Drosophila melanogaster; At, Arabidopsis thaliana. The secondary structure depictions of WTAP-a and WTAP-b are shown in the top and bottom panels. Conserved and similar residues are boxed with red ground and red font, respectively. f Intermolecular contacts between WTAP-a and WTAP-b around L3/L3’, corresponding to the orange box in b and c. g Sequence alignment of the WTAP homodimer (160–185) containing the L3/L3’ region, marked as e.