Abstract
In vitro or animal models have been used to investigate the pathogenesis of Helicobacter pylori infection. However, extrapolation to humans of results obtained with these heterologous models remains difficult. We have developed a new model for the study of H. pylori infection that uses human entire embryonic stomachs engrafted in nude mice. At 80 days after implantation, 22 of these xenografts, which exhibited a mature gastric epithelium, were inoculated with 107 to 108 CFU of either H. pylori LB1, a freshly isolated H. pylori strain (n = 12), or H. pylori ATCC 49503 (n = 10). After 12-week examination, H. pylori LB1 persistently colonized the antrum of all inoculated grafts, as assessed by culture (mucus and mucosa), immunohistochemistry (mucosa), and a rapid urease test (mucus). H. pylori ATCC 49503, either before or after in vivo passage, permitted only a transient 2-week colonization in one of the five inoculated grafts in both groups. Colonization was always associated with an increase of gastric juice pH. A mild neutrophil infiltration of the gastric mucosa was noted solely in infected grafts. Transmission electron microscopy showed adherence of H. pylori organisms to epithelial cell surface. In six animals, intracytoplasmic location of this bacterium was observed in the antrum or the fundus. These results allow us to propose this model as a new ex vivo model for the study of specific H. pylori-gastric cell interactions.
Helicobacter pylori is today recognized as a major cause of gastroduodenal diseases, including chronic gastritis and peptic ulcer, and as a risk factor for gastric carcinoma (6). Several experimental models have been developed in vitro or in animals for the study of the pathophysiology of this infection and/or for antibacterial compound screening (7, 14, 15, 19, 22–24). On one hand, in vitro models present a way to control easily the experimental parameters. However, these models rely on the use of either nonhuman epithelial cells or human cells derived from carcinomas of gastric or other origin. Thus, the extrapolation to humans of results remains difficult. On the other hand, animal models are not always optimal for pathophysiological studies, because one cannot assume that host-pathogen interaction will mimic the one observed in humans. Thus, the grafting of human tissue in mice is an interesting approach (13, 28, 31). However, only nude or severe combined immunodeficient mice are suitable for engraftment, as they have no functional T-cell lines. Since grafting of normal adult tissues except human skin (29) has been unsuccessful in nude mice, the only studies so far published rely on human embryonic or fetal tissues (13, 28, 31, 34). In our experience, human adult gastric tissues always degenerate after implantation in nude mice (unpublished data). In contrast, human embryonic stomachs can be grafted as a whole (26). Eighty days after grafting, these xenografts have grown into small organs which present all characteristics of maturity. At this time, their histological structure is comparable to what is observed in adults, and acid secretion results in an intraluminal pH of 1.5 to 3. In situ hybridization studies showed that the gastric epithelial and muscular cells were of human type whereas the vascular endothelial cells and some fibroblasts were of murine origin. We have developed a new model for the study of H. pylori infection that uses these xenografts. To this end, we inoculated, at 80 days after implantation, human embryonic stomachs with either H. pylori ATCC 49503 or a freshly isolated strain (H. pylori LB1), and monitored colonization for 3 months, using the rapid urease test, culture, histology, and transmission electron microscopy.
(This work was presented in part at the 98th General Meeting of the American Society for Microbiology, Atlanta, Ga., 17 to 21 May 1998.)
MATERIALS AND METHODS
Animals.
Twenty-five pangenic, 6- to 8-week-old, Swiss nude mice (mean weight ± standard deviation, 23 ± 5 g) purchased from Iffa Credo (Lyon, France) were used. Mice were housed in individual cages, fed with a commercial rodent diet, and given water ad libitum. All animal experimentation was performed in accordance with institutional guidelines and were approved by the Service Vétérinaire de la Santé et de la Protection Animale (Direction Générale de l’Alimentation du Ministère de l’Agriculture et de la Forêt).
Human stomachs.
Human embryonic organs of 6 to 8 weeks of gestational age were obtained after legal abortion. The embryonic stomachs, if present in the aspirated tissues, were stored at 4°C in a sterile isotonic glucose solution and grafted within 4 h. Procurement of these embryonic tissues was performed in accordance with the requirements concerning the use of human material (Avis no. 1 du Comité Consultatif National d’Ethique) and with the approvement of the French National Consultative Ethical Committee. Twenty-five entire stomachs were grafted onto 25 nude mice.
Grafts.
Mice were anesthetized with ketamine hydrochloride (10 mg/kg of body weight) intraperitoneally, which provided suitable anesthesia for 40 to 60 min. Anesthesia could be prolonged as required by repeated administration of ketamine (one-fourth of the initial dose every 20 min). Animals were attached in dorsal decubitus to plastic boards by using adhesive tips, and their abdominal skin was disinfected with 1% eosin in absolute ethanol. Then mice were placed in a sterile environment and subjected to surgery under aseptic and microsurgical conditions. The skin of the abdominal wall was opened on the midline by a xiphopubic incision and then loosened from the underlying musculoaponeurotic layer. The anterior aponevrosis was opened, and the musculus rectus abdominis was detached from the epigastric vessels and the parietal peritoneum. A pouch was built up between the epigastric vessels and the parietal peritoneum at the back and the abdominal muscle layer in front. The entire stomach, which measured about 3 by 2 by 1 mm (Fig. 1A), was introduced in this cavity in such a way that its back was in close contact with the epigastric vessels. The graft was stitched to the peritoneum by three 10/0 sutures (Fig. 1B). The pouch was closed over the flattened gastric graft by three 6/0 sutures. Finally, the skin was closed with a 5/0 uninterrupted suture. All 25 implanted stomachs were successful.
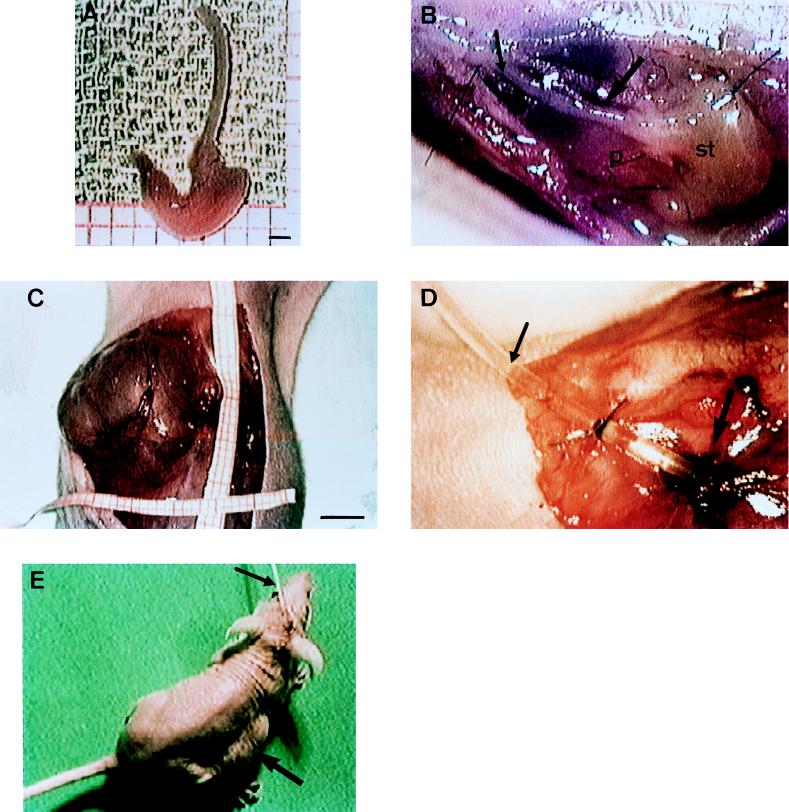
FIG. 1.
Graft implantation and catheterization. (A) Entire embryonic stomach of 8 weeks of gestational age. Bar = 1 mm. (B) Just engrafted stomach (st) with esophagus (small arrow) stitched to the peritoneum (p) and in close contact with the epigatric vessels (large arrow). (C) Xenograft exposed after incision of the abdominal skin (3 months after engraftment). Bar = 5 mm. (D) Catheter (small arrow) implanted in a mature xenograft (large arrow). (E) Nude mice with a catheter (small arrow) coming out at the nape of the neck. A tumefaction corresponding to the xenograft is visible on the right flank (large arrow).
Eighty days after implantation, mice were anesthetized again as described above. The abdominal skin was disinfected and then opened. The human stomach, which measured at this time about 2 by 2 by 3 cm (Fig. 1C), was punctured, and the gastric juice was aspirated. The gastric wall was opened, and a reference biopsy was taken for histological examination (hematoxylin-eosin) to ensure that all grafts exhibited human mature gastric epithelium as previously described (26). A Silastic catheter with an outer diameter of 600 μm (Lambert Rivière, Fontenay-sous-Bois, France) and a silicone disc attached at its proximal extremity was introduced in the stomach, which was then closed by four 6/0 interrupted sutures (Fig. 1D). The catheter was slid under the thoracic skin and came out at the nape of the neck, to which it was securely attached (Fig. 1E). The observance of rigorous standards of hygiene permitted maintenance of the catheter for 3 months. Thus, gastric juice aspiration could be performed through the catheter twice a day during the whole experimental time to avoid gastric fistulization.
Bacterial strains and growth conditions.
H. pylori LB1 and H. pylori ATCC 49503 were used for graft inoculation. H. pylori LB1 was isolated from gastric biopsies from a patient with duodenal ulcer and severe gastritis. It was originally isolated and maintained on Columbia agar (bioMérieux, Marcy l’Etoile, France) supplemented with 10% horse blood (bioMérieux), vitamin K (1.3 μg/ml; Sigma Chemical Co., St. Louis, Mo.), hemin (10 μg/ml; Sigma), and triphenyltetrazolium chloride (40 μg/ml; Prolabo, Paris, France) and containing polymyxin B (2500 UI/ml; Sigma), vancomycin (10 μg/ml; Sigma), trimethoprim (5 μg/ml; Roche, Neuilly-sur-Seine, France), and amphotericin B (10 μg/ml; Bristol-Myers Squibb, Paris, France). This strain was identified as H. pylori on the basis of Gram stain and oxidase, catalase, and urease production and as a cagA-negative (cytotoxin-associated gene A) vacA+ (vacuolating cytotoxin gene A) Tox− (vacuolating cytotoxin) strain by PCR amplification using cagA- and vacA-specific probes (1, 17) and cytotoxin assay performed as described by Figura et al. (9). The cagA+ vacA+ Tox+ status of H. pylori ATCC 49503 was similarly assessed. Further subcultures of H. pylori LB1 and H. pylori ATCC 49503 as well as subsequent isolations of these strains from grafts were performed on the selective medium described above. However, to detect contamination by other organisms, the same medium without selective mixture was used for primary culture of graft samples. All plates were incubated in a microaerobic atmosphere consisting of 80% N2, 15% CO2, and 5% O2 (IG 150 incubator; Jouan, St. Herblain, France) at 37°C for 5 days for isolation or initial culture and for 2 to 3 days for subsequent culture. Strains were stored at −80°C in brucella broth (Oxoid, Basingstoke, England) containing 15% (wt/vol) glycerol.
Bacterial inoculation.
One to three days after catheter implantation, bacterial challenge was performed. The catheterized graft of each animal was aspirated, and gastric juice was sampled for pH determination (pHG-1 pHmeter; Physitemp Instruments Inc., Clifton, N.J.). This permitted us to ensure that the gastric juice was acid, since the pH ranged from 1.5 to 2.5 for all grafts studied.
First, two grafts were inoculated, through the gastric catheter, two times at 3-day intervals with 0.6 ml of bacterial suspension (approximately 108 organisms/ml in tryptose soy broth [Oxoid]) of H. pylori LB1 obtained after two in vitro passages (initial culture followed by one subculture). Ten other grafts were later inoculated with H. pylori LB1 obtained after isolation from the initially inoculated stomachs followed by further subcultures and storage at −80°C (four in vitro passages). These in vitro passages were necessary to ensure that the strain was in pure culture and to provide sufficient bacterial material for further inoculation. It was thought that this inoculation protocol would provide the optimum opportunity for the bacteria to colonize the gastric tissue, since it has been suggested that multiple inoculations as well as the use of strains with no or only few in vitro passages may enhance the likelihood of colonization in rodents (15, 22, 24) as well as in humans (25). In the same way, five grafts were inoculated with H. pylori ATCC 49503, and subsequent inoculations were carried out in five additional grafts with the same strain obtained after isolation from primary inoculated grafts (four in vitro passages). Sterile tryptose soy broth was administered in three grafts included as controls.
Evaluation of infection.
At 2, 4, 8, and 12 weeks after inoculation, each animal was anesthetized as described above. After disinfection and incision of the abdominal wall, each graft was microsurgically opened and gastric juice was taken for pH determination. Mucus was sampled for culture and rapid urease test. Three large gastric biopsies (3 by 3 by 1 mm) were then taken from adjacent sites in one gastric area at least, for bacterial culture, histology, and electron microscopy. The gastric mucosa and then the muscular layer were stitched with five to seven 6/0 interrupted sutures. Finally, the abdominal wall was closed. No animal died from these repeated anesthetizations and surgeries.
Mucus samples were immediately plated onto both selective and nonselective agar media and also placed into a 2% urea-buffered broth for rapid detection of H. pylori urease activity. This test was read within 3 h. One biopsy specimen was fixed in 10% (wt/vol) buffered formalin (16 to 24 h) for histological examination. The second was fixed at 4°C, in 0.1 M cacodylate buffer (pH 7.4) containing 2.5% (vol/vol) glutaraldehyde for 2 h, and then in 0.1 M cacodylate buffer overnight, for electron microscopic study. The third was weighed and immediately placed in a semisolid agar transport medium (Portagerm pylori; bioMérieux) for culture. This sample was transferred to 0.5 ml of brucella broth (Difco, Detroit, Mich.) and homogenized for 1 min with an Ultra Turrax grinder (Labo-Moderne, Paris, France) before inoculation onto selective and nonselective agar. Serial dilutions of the homogenate were performed in sterile 0.9% NaCl, and 0.1-ml aliquots of the dilutions were plated onto the selective medium. Bacterial counts were expressed as CFU per gram of tissue.
Formalin-fixed specimens were processed by standard methods, embedded in paraffin, sectioned, stained with hematoxylin-eosin, and examined for histopathological changes without prior knowledge of challenge status. The intensity of antral gastritis was evaluated as previously described (20). Briefly, inflammation (number of whole inflammatory cells) and activity (number of polymorphonuclear leukocytes) were scored separately on the extent of inflammatory cell infiltration as absent, mild, moderate, or severe and classified in four different grades (0 to 3). Follicular gastritis was similarly scored on the basis of the additional presence of lymphoid follicles. The antral or fundic origin of the biopsies was verified by histological examination. For immunohistochemical examination, sections were deparaffinized through several washes with toluene and graded ethanols. Slides were then dipped in a pressure cooker filled with 10 mM citrate buffer (pH 6.0), processed until whistling occurred, cooled for 15 min at room temperature, and rinsed in distilled water. Sections were washed in Tris-HCl buffer (100 mM Tris-HCl, 100 mM NaCl [pH 7.6]) containing 0.05% (vol/vol) Tween 20 (buffer A) and then incubated with a rabbit polyclonal anti-H. pylori antibody (Dako, Copenhagen, Denmark) diluted 1/100 (vol/vol) in Tris-HCl buffer containing 0.05% Tween 20 (vol/vol) and 0.3% (wt/vol) bovine serum albumin or with Tris-HCl buffer alone as control for 30 min at room temperature. Slides were rinsed in buffer A and then treated with a biotinylated anti-rabbit antibody (Dako) diluted 1/150 (vol/vol) in buffer A for 30 min at room temperature. The sections were then washed in buffer A, and endogenous peroxidase activities were blocked by incubation for 10 min in a hydrogen peroxide solution (peroxidase-blocking solution; Dako). Thereafter, a streptavidin-horseradish peroxidase complex (Dako) diluted 1/150 (vol/vol) in buffer A was applied to all slides for 30 min at room temperature. The peroxidase-diaminobenzidine method was used for colorization, and sections were counterstained with hematoxylin.
For transmission electron microscopy (TEM), biopsy specimens were postfixed in 0.1 M cacodylate buffer containing 1% (wt/vol) osmium. They were dehydrated in increasing concentrations of ethanol (30, 50, 70, 80, and 90% [5 min for each step]; 100% [20 min, three times]) and three changes of propylene oxide (20 min for each step). Next, they were placed in a propylene oxide-epoxy resin (Epikote-812; Consortium International Pharmaceutique et Chimique, Paris, France) (vol/vol) mixture for 1 h at room temperature before being placed in 100% epoxy resin overnight as described by Luft (21). Finally, they were embedded in fresh epoxy resin for 3 days at 56°C. Thin sections (1 μm) were stained with azure blue II. Ultrathin sections of selected areas were cut on a Reichert OMU3 ultramicrotome using diamond knives, collected on copper grids, and stained with uranium acetate and Reynold’s lead citrate. For each biopsy, 16 sections were examined in a Philips CM12 transmission electron microscope at an accelerating voltage of 80 kV.
RESULTS
Colonization efficiency.
H. pylori LB1 colonized all inoculated grafts for the 3-month period of the study, as shown by bacterial culture (Table 1). Despite variation in mucosal concentrations of bacteria in the grafts and with time, each culture was always positive. Colonization became stable after 8 weeks at a level about 10 to 100 times higher than that at 2 weeks (Table 2). The colonization efficiency of H. pylori LB1 was not altered by the storage and in vitro passages. In contrast the reference strain colonized transiently only one graft of five. Bacteria were recovered only at 2 weeks and at low concentration (3 × 103 CFU/g). In vivo passage did not improve the colonization capacity of the strain, as results were of the same order as for the first inoculation. The only transiently colonized graft had a mucosal bacterial concentration of 5 × 103 CFU/g. No organism other than H. pylori was recovered on nonselective agar after culture of either mucus or mucosal samples obtained at any time from all grafts studied. Culturing of gastric mucosa was the most sensitive method (Table 1), permitting quantification of the bacterial colonization at each time point.
TABLE 1.
Colonization of human gastric xenografts by H. pylori LB1 and H. pylori ATCC 49503
| H. pylori strain used in inoculation | Time (wk) after inoculation | No. of grafts positive/total no. of grafts studied
|
|||
|---|---|---|---|---|---|
| Mucus
|
Antral mucosa
|
||||
| Rapid urease test | Culture | Immuno-histochemistry | Culture | ||
| LB1a | 2 | 2/2 | 2/2 | 2/2 | 2/2 |
| 4 | 2/2 | 2/2 | 2/2 | 2/2 | |
| 8 | 1/2 | 1/2 | 2/2 | 2/2 | |
| 12 | 1/2 | 1/2 | 2/2 | 2/2 | |
| LB1b | 2 | 10/10 | 9/10 | 8/10 | 10/10 |
| 4 | 8/10 | 8/10 | 8/10 | 10/10 | |
| 8 | 6/10 | 6/10 | 9/10 | 10/10 | |
| 12 | 8/10 | 8/10 | 10/10 | 10/10 | |
| ATCC 49503c | 2 | 1/5 | 1/5 | 1/5 | 1/5 |
| 4 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 8 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 12 | 0/5 | 0/5 | 0/5 | 0/5 | |
| ATCC 49503d | 2 | 1/5 | 1/5 | 1/5 | 1/5 |
| 4 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 8 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 12 | 0/5 | 0/5 | 0/5 | 0/5 | |
Grafts were inoculated with H. pylori LB1 freshly isolated from humans (two in vitro passages).
Grafts were inoculated with H. pylori LB1 after isolation from initially inoculated grafts (four in vitro passages).
Grafts were inoculated with H. pylori ATCC 49503 before in vivo passage in graft.
Grafts were inoculated with H. pylori ATCC 49503 after isolation from initially inoculated graft (four in vitro passages).
TABLE 2.
Colonization levels in antral mucosa of human gastric xenografts after inoculation of H. pylori LB1
| Graft no. | Bacterial concn (log CFU/g of tissue) at:
|
|||
|---|---|---|---|---|
| 2 wk | 4 wk | 8 wk | 12 wk | |
| 1a | 5.30 | 6.00 | 6.84 | 6.00 |
| 2a | 5.60 | 6.47 | 7.30 | 6.78 |
| 3b | 4.84 | 5.00 | 6.74 | 7.00 |
| 4b | 5.47 | 5.00 | 5.30 | 5.47 |
| 5b | 5.17 | 7.17 | 7.30 | 7.30 |
| 6b | 4.30 | 6.74 | 7.17 | 7.30 |
| 7b | 4.47 | 7.30 | 7.47 | 7.30 |
| 8b | 4.47 | 6.54 | 6.90 | 7.00 |
| 9b | 5.39 | 6.38 | 7.00 | 7.00 |
| 10b | 4.60 | 7.00 | 6.90 | 7.30 |
| 11b | 5.39 | 6.30 | 7.00 | 7.00 |
| 12b | 3.69 | 5.17 | 6.90 | 6.84 |
Grafts inoculated with H. pylori LB1 freshly isolated from human (two in vitro passages).
Grafts inoculated with H. pylori LB1 after isolation from the initially inoculated xenografts (four in vitro passages).
Colonization of the stomach was related to an increase of the gastric juice pH. Each time bacteria were isolated, pH increased in the range of 5 to 7.5; it remained low when colonization failed (range, 1.5 to 2.5) as well as in control grafts (range, 1.5 to 2). Interestingly, in the two grafts transiently colonized by H. pylori ATCC 49503 at 2 weeks, the pH, which was increased (6 and 6.5) at this time, returned to a low level (range, 2 to 2.5) within 4 weeks.
Macroscopic and histopathological findings.
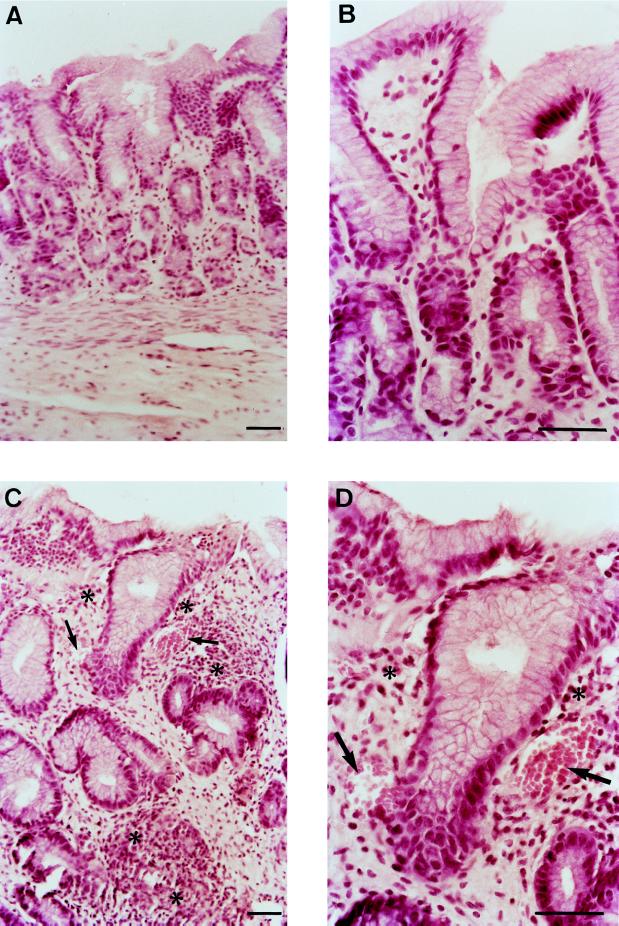
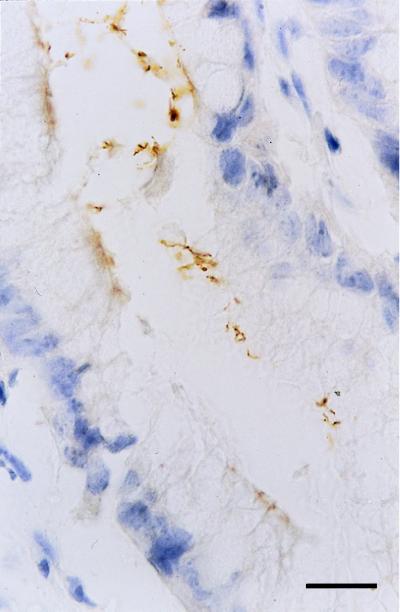
After challenge with H. pylori LB1, rare limited erythematous areas were visible at the surface of the antrum at 2 weeks. Such lesions were widespread and associated with antral hemorrhagic points from week 4. No gastric erosions or ulcerations were noted at any time after challenge. Histological examination of antral biopsies showed an inflammation/activity score of 1/0 at 2 weeks. From 4 weeks to 3 months, mild inflammation and activity (score, 1/1) were observed (Fig. 2C and D). Mucosal edema was also present and associated with capillary dilatation. Lymphoid follicles were never seen. Immunohistochemistry revealed the bacterium in the surface and pit mucus, close to the epithelial cells (Fig. 3). All of these macroscopic and microscopic findings were noted for all H. pylori LB1-infected grafts. In additional biopsies, retrospectively assessed as being of fundic origin, H. pylori was rarely observed within gastric pits. For the two grafts which were transiently colonized with the reference strain, macroscopic and histological features were the same as those found at 2 weeks for the LB1 strain. In control grafts and in stomachs inoculated with H. pylori ATCC 49503 from which no bacteria was isolated, macroscopic and histological examinations revealed no abnormalities (Fig. 2A and B).
FIG. 2.
Hematoxylin-and-eosin-stained sections of human gastric mucosa from uninfected and infected xenografts at 12 weeks after inoculation with H. pylori LB1. (A and B) Normal gastric antral mucosa. (C and D) Gastric infected mucosa, showing dilated capillaries (arrows) and mild infiltration of mononuclear cells and rare polymorphonuclear leukocytes (∗). Bars = 50 μm.
FIG. 3.
H. pylori bacteria at the surface of mucus cells in the gastric antral mucosa from an infected xenograft (immunohistochemical peroxidase staining). Bar = 10 μm.
TEM findings.
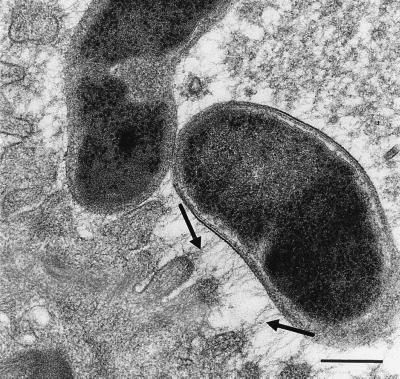
Ultrastructural study confirmed the presence of H. pylori at the surface of the mucosa in 38 of the 48 antral biopsies from the H. pylori LB1-infected stomachs and in one of the two antral biopsies from the grafts infected with the reference strain. These bacteria were frequently associated with the mucosal cell surface. In most of these cases, filamentous strands joining the bacterial membrane and the epithelial cell membrane were observed (Fig. 4). In 6 of the 16 fundic biopsies taken from four H. pylori LB1-infected grafts, bacteria were observed. They were close to parietal cells and within secretory canaliculi at 2 weeks in two grafts and at 4 weeks in one graft (Fig. 5). No organisms were subsequently observed at this location despite a thorough search. In both sites, no preferential site of adherence was noted, and in particular no accumulation of bacteria was noted at intracellular junctions. In a few grafts infected with H. pylori LB1, intracellular bacterial localization could be seen. Intracytoplasmic bacteria were observed in antral mucus cells of three grafts at 4 weeks (n = 2) and 8 weeks (n = 2) and in parietal cells in three other grafts at 2, 4, and 8 weeks (Fig. 6). These bacteria were not surrounded by a cell membrane system. Extracellular as well as intracellular organisms appeared either as cross-sectioned bacteria or as curved bacilli with occasionally transversally or longitudinally sectioned flagella.
FIG. 4.
Transmission electron micrograph showing the attachment of H. pylori organisms to the surface of gastric epithelial cells with filamentous strands between bacterial membrane and cytoplasmic membrane (arrows). Bar = 0.25 μm.
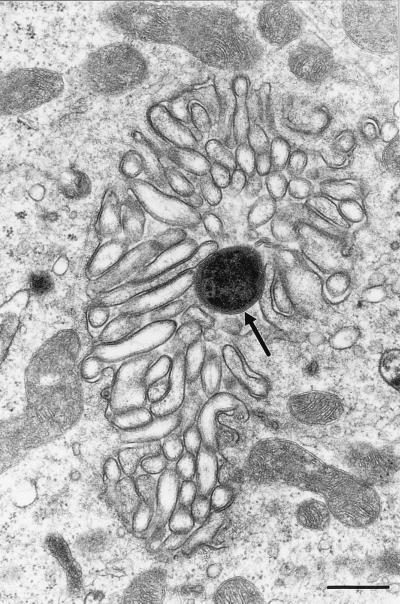
FIG. 5.
Transmission electron micrograph showing H. pylori organism (arrow) in the canalicular system of a parietal cell. Bar = 0.5 μm.
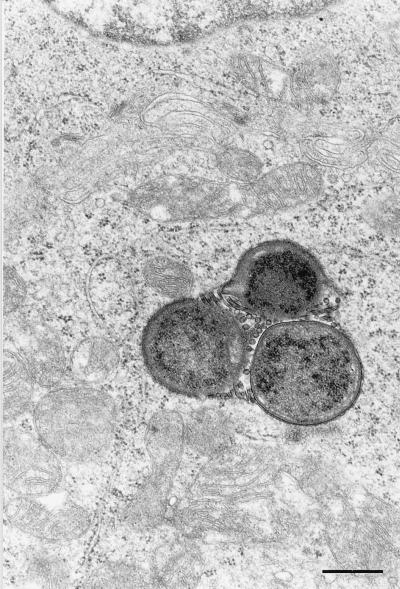
FIG. 6.
Transmission electron micrograph showing cross-sectioned H. pylori with sectioned flagella in the cytoplasm of a parietal cell. Bar = 0.5 μm.
DISCUSSION
Human embryonic stomachs implanted into nude mice provide a suitable model for the study of H. pylori infection, as a persistent colonization of the gastric mucosa may be obtained with gastric bacterial densities comparable to what is observed in humans (2). A sustained infection has been achieved only with a freshly isolated strain. The laboratory strain (H. pylori ATCC 49503) seldom colonized grafts and was not detected in the gastric environment for more than 2 weeks even after an in vivo passage. Such failure may be due to bacterium- and/or host-dependent factors. It has been suggested that the use of fresh H. pylori isolates may be necessary to obtain colonization of the gastric mucosa in mice (15, 22) as well as in humans (25). However, as we have inoculated only one fresh isolate and one reference strain, further studies, using more laboratory strains and freshly isolated strains with different virulence phenotypes, are necessary to elucidate this point in our model.
Acute or active chronic gastritis in H. pylori-infected patients is characterized by the presence of numerous neutrophils (10). In the present study, infection, once established, induced only a limited neutrophil inflammatory response. Similar findings have been noted in immunocompetent H. pylori-infected mice (8, 19). It has been suggested that such an inflammatory response should be due predominantly to host-dependent factors responsible for modulation of the immune response (8). In our model, the infected tissue is of human origin whereas inflammatory cells are of murine origin. Thus, it is conceivable that the limited neutrophil reaction may be, at least partially, due to host factors. The absence of lymphoid formation may be explained by the immunodeficient status of the nude mice, which may also have facilitated the establishment of persistent colonization.
We have rarely observed intimate contact between bacterial cell wall and the epithelial cell surface. However, filamentous strands between bacterial membrane and epithelial cell membrane were readily observed. This may be due to an interaction between the bacterial glycocalyx and surface polysaccharides of the epithelial cells. Although H. pylori is considered an extracellular pathogen, intracellular localization of this bacterium in gastric epithelial cells from humans with chronic gastritis or gastric metaplasia has been described (3, 4, 16, 27, 33). In the present study, internalization of H. pylori was observed in a few cases. In TEM studies, tangential sections may be responsible for images appearing falsely to show intracellular localization. This may be eliminated in our study since internalized bacteria were never surrounded by a trilamellar membrane. In contrast to other studies (4, 16, 33), intracellular H. pylori were observed neither inside nor closely associated with intracytoplasmic vacuoles or lysosomes. Intracanalicular localization of this bacterium has been rarely found in humans (5), whereas internalization of H. pylori in human parietal cells has not been described. The secretory canaliculus may represent a site of penetration into parietal cells. However, further studies are needed to elucidate this point since images showing engulfment have never been observed in our study. The significance of the intracytoplasmic location of H. pylori in gastric epithelial cells is not known. It may represent a way for this organism to escape the immune response and may also explain the failure to eradicate bacteria when antibiotics with poor intracellular diffusion are administered, provided the organism maintains its ability to multiply and to evolve toward extracellular organisms able to colonize the epithelium.
In humans, acute H. pylori infection induces transient hypochlorhydria, the duration of which may reach several months (11, 25, 30). However, the mechanism of this phenomenon is unknown. One hypothesis is that the gastric acid may be neutralized by ammonia, generated by the H. pylori urease. This represents a possible mechanism in our model since the concentrations of urea in the gastric juice of grafts were similar (4 to 6 mmol/liter [unpublished data]) to those observed in humans (18). It has also been suggested that H. pylori may induce directly or indirectly a decrease in the secretory activity of the gastric parietal cells and consequently hypochlorhydria (12, 32). In the present study, parietal cells in a poorly secreting or nonsecreting state, as found by Graham et al. (11) in the gastric mucosa of a patient with acute H. pylori gastritis, were not observed.
Catheterization of grafts allowed us to prevent fistulization which would occur in case of high intraluminal pressure. The catheter may also be used for continuous sampling of mucus. This should represent a convenient way for studying the progress of infection. However, for the detection of H. pylori, the culture and/or the urease test performed on mucus samples proved less sensitive than culture of gastric mucosa. Size of the biopsy may explain the constant recovery of bacteria by culture from implanted stomachs. Thus, microsurgery remains necessary for optimal follow-up.
Altogether, this animal model presents several advantages. The colonization by H. pylori strains can be controlled and followed up, and the consequence of infection on the mucosa can be assessed. This model should allow a more extensive and specific study of H. pylori infection. Moreover, since bacterial counts are easy to obtain, it may also be suitable for the study of in vivo activity of antimicrobial agents.
ACKNOWLEDGMENTS
We thank Nicole Lubraniecki and Monique Simonetti for technical assistance.
REFERENCES
- 1.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Tham K T, Peeks R M, Jr, Cover T L, Blaser M J. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 3.Bale G, Malfertheiner P, Ditschuneit H. Campylobacter-like organisms in the duodenal mucosa in patients with active duodenal ulcer. Klin Wochenschr. 1987;65:144–146. doi: 10.1007/BF01728609. [DOI] [PubMed] [Google Scholar]
- 4.Bode G, Malfertheiner P, Ditschuneit H. Pathogenetic implications of ultrastructural findings in Campylobacter pylori related gastroduodenal disease. Scand J Gastroenterol. 1990;23(Suppl. 142):25–39. [PubMed] [Google Scholar]
- 5.Chen X G, Correa P, Offerhaus J, Rodriguez E, Janney F, Hoffmann E, Fox J, Hunter F, Diavolitsis S. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am J Clin Pathol. 1986;86:575–582. doi: 10.1093/ajcp/86.5.575. [DOI] [PubMed] [Google Scholar]
- 6.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauchère J L. In vivo and in vitro models of Helicobacter pylori infection. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 141–157. [Google Scholar]
- 8.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect Immun. 1998;66:1349–1355. doi: 10.1128/iai.66.4.1349-1355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figura N, Gugliemetti P, Rossolini A, Barberi A, Cuzi G, Musmanno R A, Russi M, Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989;27:225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fléjou J F. Histological diagnosis of Helicobacter pylori infection. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 235–247. [Google Scholar]
- 11.Graham D Y, Lesley M D, Alpert C, Smith J L, Yoshimura H H. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol. 1988;83:974–980. [PubMed] [Google Scholar]
- 12.Healey Z, Calam J. Inhibiting acid and Helicobacter pylori. Gut. 1997;41:125–126. doi: 10.1136/gut.41.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang G T, Eckmann L, Savidge T C, Kagnoff M F. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM-1) expression and neutrophil adhesion. J Clin Investig. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabir A M A, Aiba Y, Takagi A, Kamiya S, Miwa T, Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997;41:49–55. doi: 10.1136/gut.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karita M, Kouchiyama T, Okita K, Nakazawa T. New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroenterol. 1991;86:1596–1603. [PubMed] [Google Scholar]
- 16.Kazi J L, Sinniah R, Zaman V, Ng M L, Jafarey N A, Alam S M, Zuberi S J, Kazi A M. Ultrastructural study of Helicobacter pylori-associated gastritis. J Pathol. 1990;161:65–70. doi: 10.1002/path.1711610111. [DOI] [PubMed] [Google Scholar]
- 17.Labigne A, Lamouliatte H, Birac C, Sedallian A, Mégraud F. Distribution of the cagA gene among Helicobacter pylori strains associated with peptic ulcer. Am J Gastroenterol. 1994;89:1326. [Google Scholar]
- 18.Labigne A. Pathogenic properties of Helicobacter pylori. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 119–139. [Google Scholar]
- 19.Lee A, O’Rourke J, Corazon De Ungria M, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 20.Lozniewski A, De Korwin J D, Conroy M C, Plénat F, Weber Mich. Evaluation of Pyloriset Dry, a new rapid agglutination test for Helicobacter pylori antibody detection. J Clin Microbiol. 1996;34:1773–1775. doi: 10.1128/jcm.34.7.1773-1775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luft J H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Washizuka Y, Matsumoto Y, Tawara S, Ikeda F, Yokota Y, Karita M. Induction of ulceration and severe gastritis in Mongolian gerbil by Helicobacter pylori infection. J Med Microbiol. 1997;46:391–397. doi: 10.1099/00222615-46-5-391. [DOI] [PubMed] [Google Scholar]
- 24.McColm A A. Nonprimate animal models of H. pylori infection. In: Clayton C L, Mobley H L T, editors. Methods in molecular medicine, Helicobacter pylori protocols. Totowa, N.J: Humana Press Inc.; 1997. pp. 235–251. [DOI] [PubMed] [Google Scholar]
- 25.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192–199. [PubMed] [Google Scholar]
- 26.Muhale F, Lozniewski A, Hatier R, Morali A, Duprez A. In vivo human parietal cell maturation: a study using a xenograft model. J Pediatr Gastroenterol Nutr. 1998;26:558. [Google Scholar]
- 27.Noach L A, Rolf T M, Tytgat G N J. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699–704. doi: 10.1136/jcp.47.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettoello-Mantovani M, Kollmann T R, Raker C, Kim A, Yurasov S, Tudor R, Wiltshire H, Goldstein H. Saquinavir-mediated inhibition of human immunodeficiency virus (HIV) infection in SCID mice implanted with human fetal thymus and liver tissue: an in vivo model for evaluating the effect of drug therapy on HIV infection in lymphoid tissues. Antimicrob Agents Chemother. 1997;41:1880–1887. doi: 10.1128/aac.41.9.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plénat F, Vignaud J M, Guerret-Strocker S, Hartmann D, Duprez K, Duprez A. Host-donor interactions in healing of human split-thickness skin grafts onto nude mice: in situ hybridization, immunochemical, and histochemical studies. Transplantation. 1992;53:1002–1010. doi: 10.1097/00007890-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Ramsey E J, Carey K V, Peterson W L, Jackson J J, Murphy F K, Read N W, Taylor K B, Trier J S, Fordtran J S. Epidemic gastritis with hypochlorhydria. Gastroenterology. 1979;76:1449–1457. [PubMed] [Google Scholar]
- 31.Seydel K B, Li E, Swanson P E, Stanley S L., Jr Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taguchi Y, Kaito M, Gabazza E C, Takaji S, Shibata T, Oka S, Ikemura N, Nakao K, Hashimoto Y, Imoto I. Helicobacter pylori inhibits the secretory activity of gastric parietal cells in patients with chronic gastritis. Scand J Gastroenterol. 1997;32:656–663. doi: 10.3109/00365529708996514. [DOI] [PubMed] [Google Scholar]
- 33.Tricottet V, Bruneval P, Vire O, Camilleri J P. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10:113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]
- 34.Winter H S, Fox C H, Hendren R B, Isselbacher K J, Folkman J, Letvin N L. Use of an animal model for the study of the role of human immunodeficiency virus 1 in the human intestine. Gastroenterology. 1992;102:834–839. doi: 10.1016/0016-5085(92)90166-v. [DOI] [PubMed] [Google Scholar]