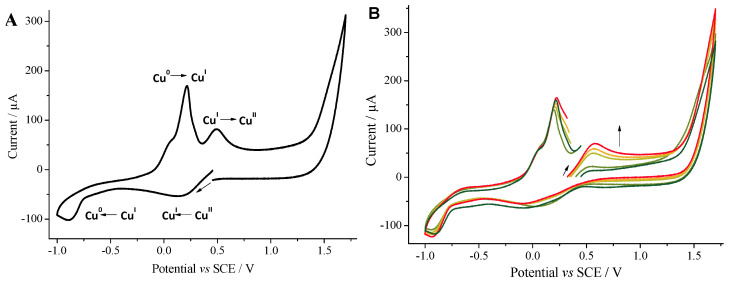
Figure 1.
(A). Cyclic voltammogram toward reduction potentials of a solution of CuIICl2 (2 mM) in MeOH (see Figure S9 for the voltammogram toward oxidation). (B). Cyclic voltammogram toward oxidation potentials monitoring the reduction in Cu(II) to Cu(I) by PhB(OH)2 in the presence of TBAOH CuIICl2 (2 mM, dark green). Addition of PhB(OH)2 (10 mM, light green). Addition of TBAOH (5 mM, apple green) at t = 0. After 20 min (light orange). After 30 min (orange). After 45 min (red). Working electrode: glassy carbon (Ø = 3 mm); scan rate: 0.5 V s−1; supporting electrolyte: nBu4NBF4 (0.3 M); recorded at ambient temperature starting at the open circuit potential (OCP).