Figure 5.
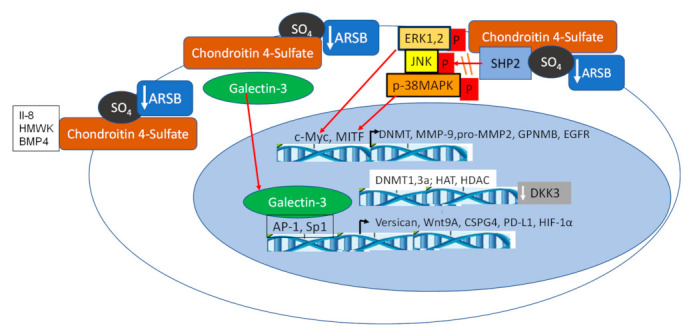
Overall schematic of transcriptional effects following decline in ARSB. Decline in ARSB leads to increase in chondroitin 4-sulfation, due to failure to remove the sulfate group at the non-reducing end and inhibition of degradation, as occurs in the inherited disorder MPS VI. Increased binding to C4S occurs for several vital molecules, including IL-8 [29] and BMP-4 [30]. Sequestration of IL-8 in the cell membrane can lead to increased neutrophil chemotaxis and contribute to impaired mucociliary clearance. BMP4 retention with C4S can alter Wnt-BMP interactions and reduce the Smad-mediated expression of CHST11 [30], thereby impairing production of new C4S. Galectin-3 binds less to more highly sulfated C4S, leading to availability for binding with the insulin receptor, and thereby contributing to insulin resistance [80,81,82]. In addition, galectin-3 acts as a co-transcriptional activator, combining with AP-1 and Sp1 for enhanced transcription of versican [37], CSGP4 [33], HIF-1α [35], Wnt9A [32], and PD-L1 [85]. In contrast, SHP2 binds more with C4S when ARSB is inhibited, leading to decline in phosphatase activity and sustained phosphorylation of important mediators, including phospho-ERK [33,36,83], phospho-JNK [28], and phospho-P38 MAPK [14,38]. Through a network of signaling events, nuclear c-Myc [36] and MITF [14] and other transcription factors act to increase expression of pro-MMP2 [33], MMP9 [33,83], GPNMB [14], EGFR [28], and DNMT1 and 3a [36]. Hence, a broad range of vital cellular processes are regulated due to changes in ARSB activity and chondroitin-4 sulfation.
