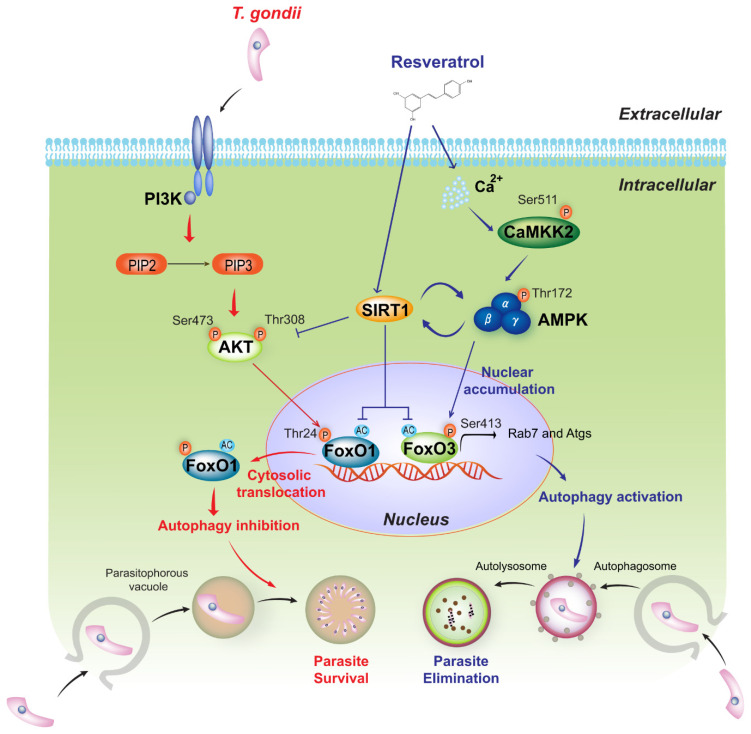
Figure 7.
Essential function and molecular mechanism of SIRT1 in modulating the host immune response to T. gondii infection. In macrophages, myeloid-specific SIRT1 increases autophagy activity during T. gondii infection, thereby eliminating intracellular T. gondii by inducing the colocalisation of parasitophorous vacuoles with autophagosomes/lysosomes. As a key regulatory mechanism of autophagy, SIRT1 attenuates the acetylation of the transcription factor FoxO1 and its PI3K/AKT-dependent phosphorylation (Thr24) in response to T. gondii, thus regulating FoxO1 transactivation activity by preventing its nuclear exclusion. Moreover, SIRT1 promotes FoxO3a deacetylation and its CaMKK2/AMPK-dependent phosphorylation (Ser413), leading to nuclear accumulation and the transactivation of FoxO3a. Pharmacological activation of SIRT1 by RES induces antiprotozoal autophagy by regulating FoxO1 and FoxO3a activity via the PI3K/AKT and CaMKK2/AMPK signalling pathways, respectively.