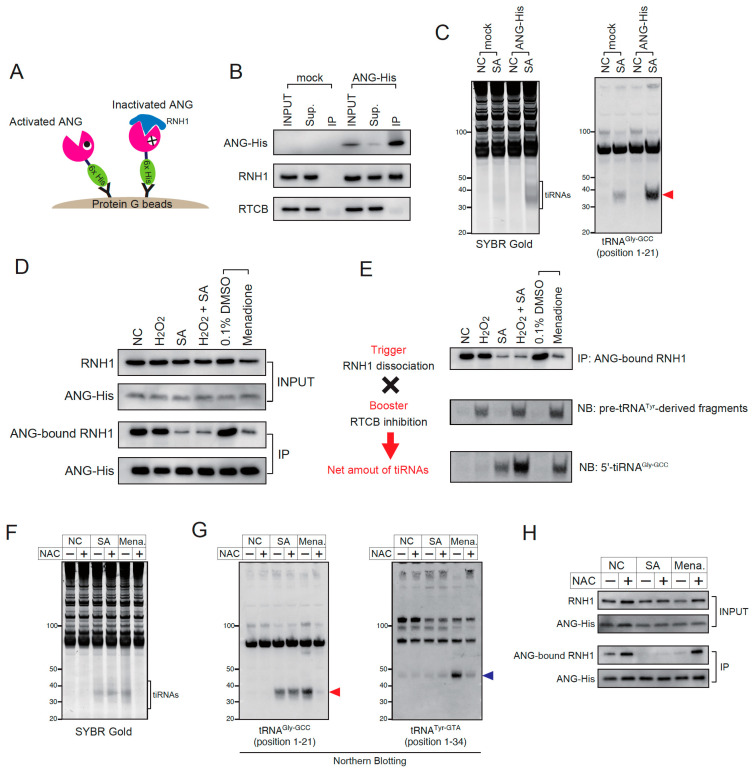
Figure 5.
Stress-induced tiRNA production is regulated by both RNH1 dissociation and RTCB inhibition. (A–D) Evaluation of stress-induced RNH1 dissociation from angiogenin. (A) Schematic model of ANG-His pulldown. When ANG was inactivated by RNH1, RNH1 is co-immunoprecipitated with ANG-His. Under the condition where ANG is activated, RNH1 is not co-immunoprecipitated because it dissociates from ANG. (B) Efficiency of immunoprecipitation. Overexpressed ANG-His was concentrated by immunoprecipitation. Under non-stress condition, RNH1 is co-immunoprecipitated with ANG-His. Note that RTCB was not co-immunoprecipitated with ANG-His, suggesting that RTCB does not interact with ANG or RNH1. (C) Overexpressed ANG-His is enzymatically functional. Overexpression of ANG-His enhanced sodium arsenite-induced tiRNA production. 5′-tiRNAGly-GCC is indicated by a red arrowhead. Note that ANG-His overexpression does not induce tiRNA production under non-stress conditions, suggesting that ANG-His is completely inhibited by RNH1 under basal conditions. (D) ANG-His pulldown under various stress stimuli. (E) The net amount of tiRNAs is regulated by both RNH1 dissociation and RTCB inhibition. RNH1 dissociation (trigger), RTCB inhibition (booster) and the net amounts of tiRNAs are evaluated by ANG-bound RNH1, pre-tRNATyr-derived fragment and 5′-tiRNAGly-GCC, respectively. (F–H) The effect of N-acetyl-L-cysteine treatment (at 5 mM) on tiRNA production. (F) SYBR Gold staining and (G) Northern blotting for tRNAGly-GCC and tRNATyr-GTA. 5′-tiRNAGly-GCC is indicated by a red arrowhead, pre-tRNATyr-GTA-derived fragments generated due to RTCB inhibition is indicated by a blue arrowhead. (H) ANG-His pulldown. Note that N-acetyl-L-cysteine treatment did not prevent RNH1 dissociation, suggesting that sodium arsenite-induced RNH1 dissociation is independent of oxidative stress. NC, negative control; SA, sodium arsenite; Mena., menadione; NAC, N-acetyl-L-cysteine.