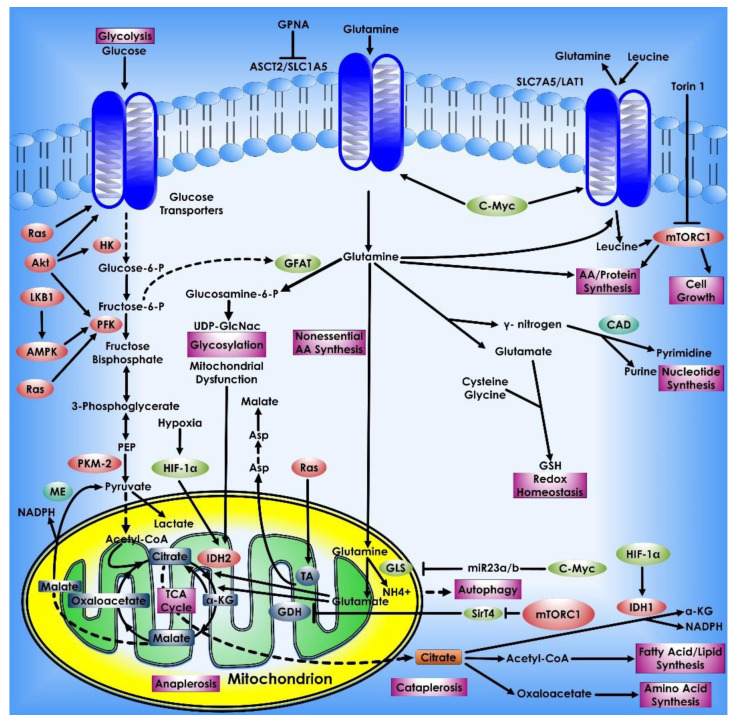
Figure 9.
Glutamine metabolism in Cancer Cells. Glutamine is a key metabolic fuel that assists rapidly growing cells in meeting the increased need for ATP, biosynthetic precursors, and reducing agents. Glutamine enters the cell via the amino acid transporter ASCT2/SLC1A5 and is transformed into glutamate in the mitochondria via a glutaminase-catalyzed deamination process (GLS). Glutamate is metabolized to the TCA cycle intermediate α-KG by GDH or alanine or aspartate transaminases (TAs), which also create the respective amino acid. α-KG is an important molecule that aids in both ATP generation and the replenishment of TCA cycle intermediates, a process known as anaplerosis. α-KG can be converted to citrate in a reductive carboxylation process mediated by IDH2 during periods of hypoxia or mitochondrial malfunction. The freshly generated citrate leaves the mitochondria and is utilized to manufacture fatty acids and amino acids as well as the reducing agent, NADPH (cataplerosis). In the cytosol, glutamine lends its (amide) nitrogen for the synthesis of nucleotides and hexosamines, resulting in the production of glutamate. Through the synthesis of glutathione, cytosolic glutamate is essential for maintaining redox equilibrium and protecting cells from oxidative damage. Many cancer cells have oncogene-dependent glutamine addictions, and glutamine can increase proliferative signaling. For example, glutamine influx via SLC1A5 is linked to efflux via the SLC7A5/LAT1 transporter, enabling leucine into the cell and activating mTORC1-mediated cell growth. Furthermore, the signaling molecules Akt, Ras, and AMPK stimulate glycolytic enzymes and promote lactate synthesis (Warburg effect), requiring cancer cells to use glutamine metabolism to fulfill higher energy needs. The proto-oncogene c-Myc promotes glutaminolysis by activating the GLS and SLC1A5 genes through transcriptional activation. Proteins that have been glycosylated by glutamine, including growth factor receptors, can be targeted to the cell surface and activated.