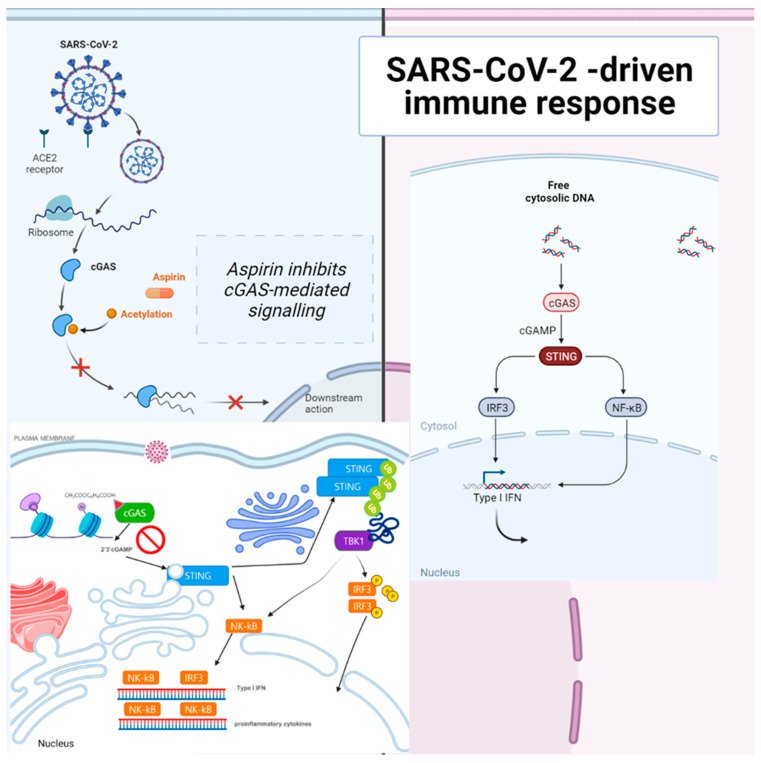
Figure 3.
Aspirin strongly inhibited cGAS-mediated TBK1–IRF3 signaling. The sensing of cytosolic DNA by cGAS is central to the pathogenesis of several autoinflammatory syndromes and some autoimmune diseases, such as systemic lupus erythematosus (SLE), because the presence of DNA in the cytoplasm is usually a sign of microbial infections and is quickly detected by cGAS, eliciting anti-infection immune responses. However, the chronic activation of cGAS by self-DNA leads to severe autoimmune diseases for which no effective treatment is yet available. The activation of cGAS signaling requires its deacetylation, while acetylation inhibits cGAS activation, and the compulsory acetylation of cGAS by aspirin forcefully suppresses self-DNA–induced autoimmunity, giving aspirin therapeutic potential in this context. Recently, the unique role of the cGAS–stimulator of the STING pathway in orchestrating innate and adaptive immunity has led to vast interest in employing antagonists of the cGAS–STING pathway as SARS-CoV-2 therapeutic adjuvants. The DNA sensor cGAS detects pathogenic or cytosolic nucleic acids and produces the second messenger, 2′3′-cGAMP, which binds to STING. Activated STING dimerizes and translocates from the endoplasmic reticulum (ER) to the Golgi apparatus. STING is ubiquitinated at the Golgi apparatus, an anchor for TBK1. Stimulation of the cGAS–STING pathway by cytosolic nucleic acids can activate interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), and promote the transcription of type I interferons (IFNs) and other proinflammatory cytokines, modulating antigen presentation and immune responses. Therefore, cGAS–STING antagonists are promising adjuvants for constructing effective SARS-CoV-2 therapeutics. Acetylation inhibits cGAS activation, and enforced acetylation of cGAS by aspirin robustly suppresses self-DNA–induced autoimmunity. Aspirin inhibited cGAS-mediated IFN production [39]. The acute or chronic use of ibuprofen and other NSAIDs was not associated with worse COVID-19 disease outcomes [50]. NSAID use might confer a modest benefit concerning survival [51]. Early aspirin prescription may be associated with lower odds of in-hospital mortality among hospitalized patients with moderate COVID-19 in 2,446,650 COVID-19–positive patients [52]. Aspirin is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized COVID-19 patients. NSAID use might confer a modest benefit concerning survival [51].