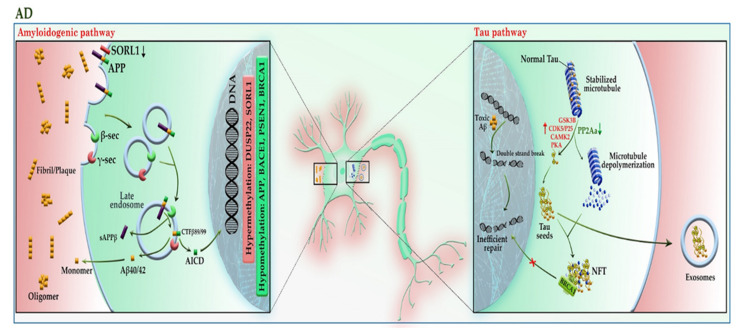
Figure 4.
A schematic representation of the selected mechanisms in the pathogenesis of AD. Under physiological conditions, APP is cleaved in the non-amyloidogenic pathway (not shown). In the absence of SORL1 due to epigenetic silencing or mutation, APP is shunted into the late endosomal pathway. In the amyloidogenic pathway, APP enters the late endosome, where it is cleaved by the β-secretase (BACE1), and then by γ-secretase. AICD enters the nucleus and acts as a transcription factor, whereas the Aβ40/42 peptides and sAPPβ are secreted to the extracellular space. An imbalance of Aβ production and its clearance from the brain promotes Aβ aggregation and deposition. The Aβ aggregates can activate the kinases involved in the tau pathway, leading to tau hyperphosphorylation. The aberrant hyperphosphorylation of tau causes p-tau to be separated from microtubules (MTs), leading to MTs depolymerization and axonal degeneration. The disruption of the tau pathway leads to the accumulation of tau aggregates to form oligomers and neurofibrillary tangles (NFTs) within neurons. The p-tau oligomers (tau seeds) can be released into the extracellular space and taken up by unaffected neurons. The tau aggregates sequester BRCA1 protein in the cytoplasm and prevent it from executing its physiological function, leading to the accumulation of DNA damage induced by Aβ. Red and green colors highlighted hypermethylation and hypomethylation, respectively. AICD: amyloid precursor protein (APP) intracellular domain; APP: amyloid precursor protein; BRCA1: breast cancer type 1; CAMK2: calcium/calmodulin-dependent protein kinase II; CDK5: cyclin-dependent kinase 5; CTF-β89/99: β-C-terminal fragment 88/99; DUSP22: dual-specificity phosphatase 22; GSK-3B: glycogen synthase kinase-3B; PKA: protein kinase A; PP2A: protein phosphatase 2; sAAPβ: soluble amyloid precursor protein β; SAM: S-adenosyl methionine; SORL1: sortilin related receptor 1; β-sec: beta-secretase 1; γ-sec: γ-secretase.