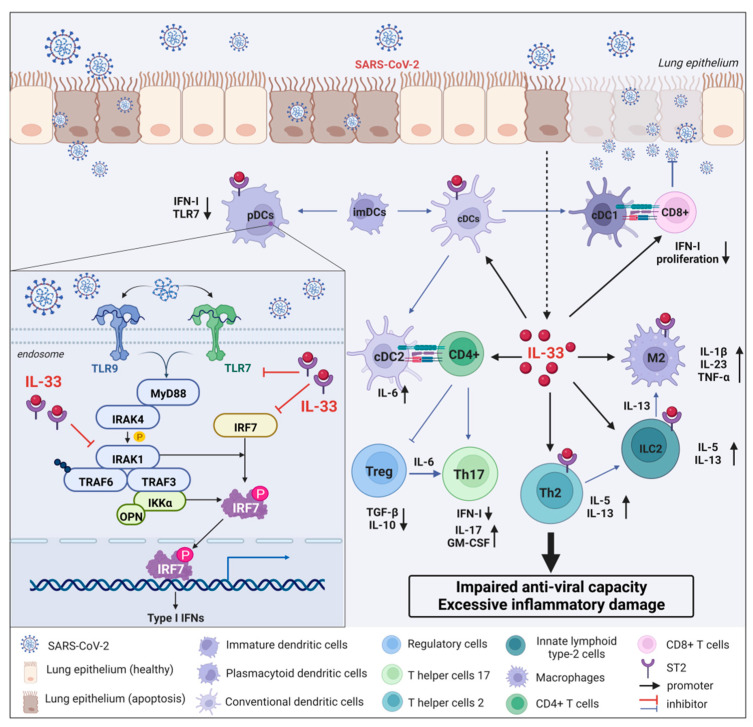
Figure 4.
Interaction mechanism between IL-33 and IFN-I released by pDCs during COVID-19 infection. After the lung epithelium is infected with SARS-CoV-2, the replicating virus can cause epithelial cell apoptosis and directly damage the epithelium. Dendritic cells present antigens to helper T cells. Immature DCs differentiate into conventional DCs (cDCs) and plasmacytoid DCs (pDCs). IFN-I specificity derived from pDCs is dependent on the Toll-like receptor-7 (TLR7)/TLR9 pathway. Upon COVID-19 infection, TLR7 or TLR9 activates MyD88 and IL-1 receptor-associated kinase 4 (IRAK-4), which then interact with tumor necrosis factor receptor-associated factor-6 (TRAF6), TRAF3, IRAK1, IKKα, and interferon regulatory factor 7 (IRF7) interaction. Ultimately, IRAK-1 and IKKα phosphorylate IRF7, leading to IRF7 activation and induction of IFN-I gene transcription and massive IFN-I production. Additionally, IL-33, an important inflammatory storm cytokine, is abundantly released from apoptotic epithelial cells and inhibits pDC-dependent IFN-I by rapidly depleting the intracellular adaptor molecules IRAK1 and viperin, resulting in a hyporesponsive state of TLR7. Meanwhile, IL-33 induces the expression of a large number of cytokines by interacting with immune cells, such as macrophages, ILC2 cells, Th2 cells, Th17 cells, and Treg cells, which ultimately leads to abnormal inflammatory damage and decreased antiviral capacity in COVID-19 patients.