Figure 2.
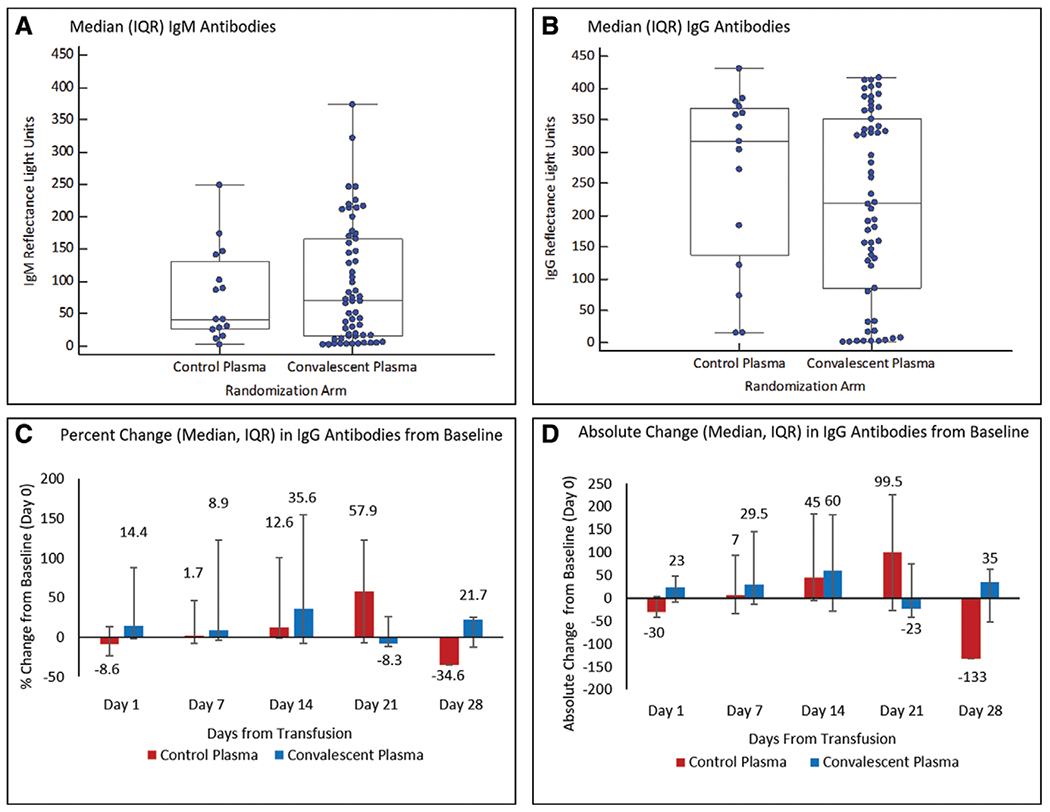
Antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in plasma recipients. Presence of immunoglobulin M (IgM) (A) and immunoglobulin G (IgG) (B) antibody levels to the nucleocapsid protein (NP) of SARS-CoV-2 in trial participants at baseline. Humans without exposure to SARS-CoV-2 typically exhibit less than 25 reflectance light units, with several hundred reflectance light units indicating a very strong antigen/antibody band. Percent (C) and absolute (D) changes in IgG antibody levels to the NP of SARS-CoV-2 at 1, 7, 14, 21, and 28 d after administration of 2 U of convalescent versus standard plasma (on day 0–baseline). Antibody levels were not measured after hospital discharge/death. IQR = interquartile range.
