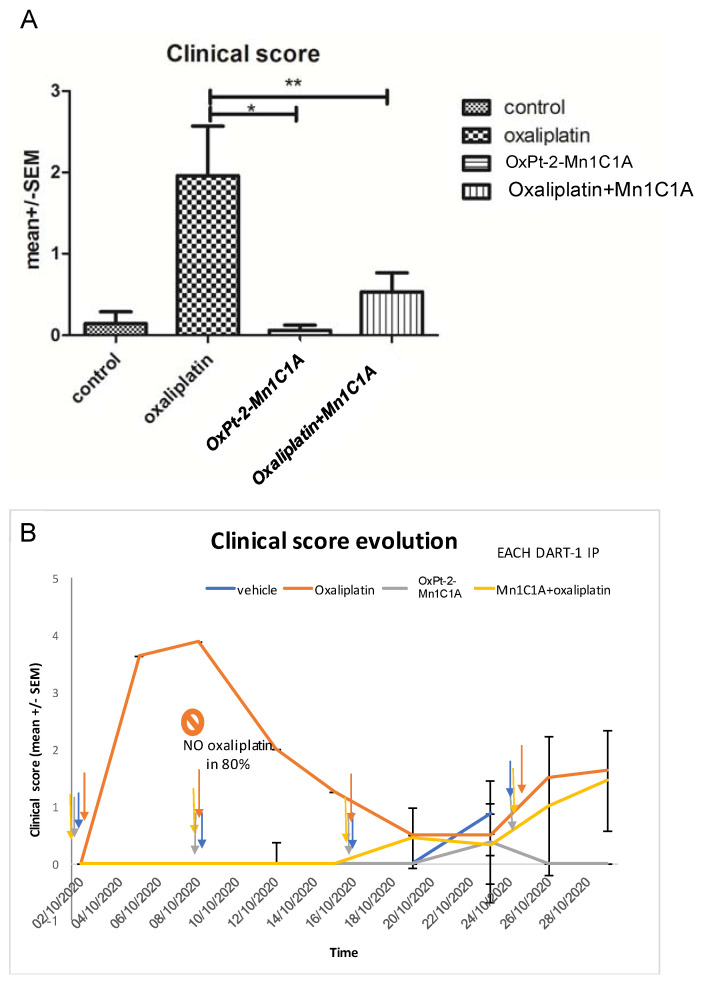
Figure 6.
Clinical score in BALB/c mice inoculated with CT26 and treated by weekly intraperitoneal injection with vehicle (8 mice), oxaliplatin (8 mice, 10 mg/kg/week), OxPt-2-Mn1C1A (9 mice, 10 mg/kg/week), and Mn1C1A + oxaliplatin (9 mice, 10 mg/kg/week). The clinical score, proportional to toxicity, was evaluated blindly in each mouse, by the investigator with four criteria: asthenia, alopecia, diarrhea, and weight loss. (A) Mean clinical score in each group depending on the treatment received. The p-values are denoted as follows: * p < 0.05 and ** p < 0.01. (B) Clinical score evolution over time. Each dart represents 1 intraperitoneal injection of each treatment. In the oxaliplatin group, 80% of the mice did not receive the second oxaliplatin IP injection due to weight loss of >20%. In the Mn1C1A + oxaliplatin and OxPt-2-Mn1C1A group, the clinical score raised with tumor growth over time but no significant toxicity was observed, and every IP injection could be received.