Table 2.
The structures and chemical yields of α-GalCer analogs (1) a.
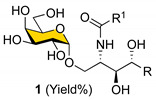
| ||||||||
|---|---|---|---|---|---|---|---|---|
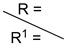
|
(CH2)5CH3 |
(CH2)
13CH3 |
(CH2)20CH3 | (CH2)4Ph | (CH2)6Ph | (CH2)9Ph | (CH2)64-CF3Ph |
(CH2)4CHCH3(CH2)
2CH(CH3)2 |
| C7H15 | 1ba (79) | -- | 1ca (72) | 1da (58) | 1ea (66) | 1fa (65) | 1ga (79) | 1ha (81) |
| C25H51 | 1bb (72) | 1a (89) | 1cb (77) | 1db (51) | 1eb (72) | 1fb (69) | 1gb (70) | 1hb (46) |
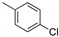
|
1bc (78) * | -- | 1cc (77) | 1dc (73) * | 1ec (78) * | 1fc (67) * | 1gc (65) * | 1hc (73) * |
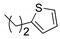
|
1bd (77) * | -- | 1cd (79) * | 1dd (75) * | -- | 1fd (76) * | 1gd (78) * | 1hd (77) * |
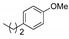
|
1be (74) | -- | 1ce (64) | 1de (50) | 1ee (73) | 1fe (75) | 1ge (69) | 1he (82) |
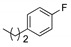
|
1bf (70) | -- | 1cf (71) | 1df (57) | 1ef (74) | 1ff (64) | 1gf (67) | 1hf (77) |
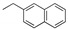
|
-- | -- | 1cg (68) | -- | 1eg (68) | 1fg (66) | 1gg (74) | 1hg (73) |
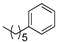
|
1bh (72) | -- | 1ch (75) | 1dh (53) | 1eh (76) | 1fh (73) | 1gh (70) | 1hh (77) |
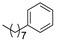
|
1bi (71) | 1c (75) | 1ci (74) | -- | 1ei (74) | 1fi (77) | 1gi (70) | 1hi (73) |
a Isolated yields are shown in parentheses based on the transformation of 33–41 to 1a–1hi (for four steps). * Obtained from the modified procedure (Scheme 8).
