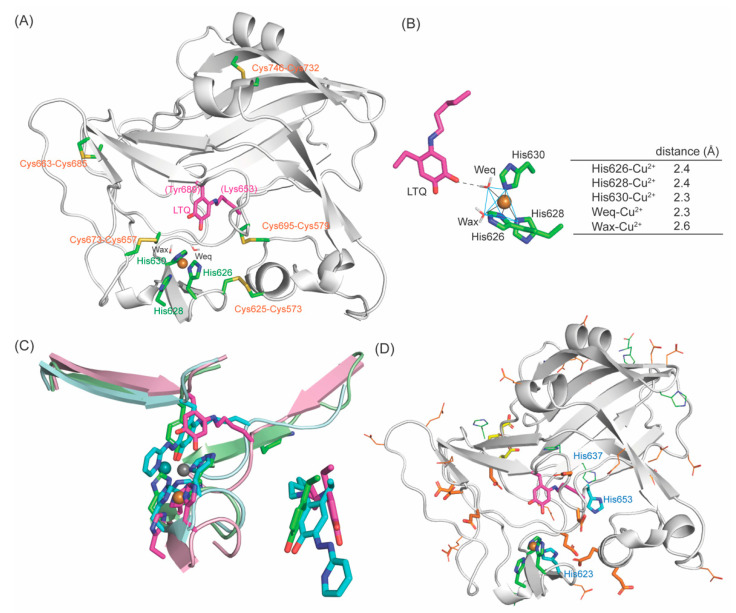
Figure 12.
A 3D–modeled structure of the resting form of LOXL2: (A) The active site structure. (B) The Cu2+–coordination environment is a slightly distorted square pyramidal with Wax–Cu2+ in a Jahn–Teller axis and Weq, His626, His628 and His630 comprise the square bottom. O4 carbonyl of LTQ is 2.7Å from Weq. (C) Left: Overlay of the resting form of LOXL2 (LTQ and three His in magenta, peptide in pink, Cu2+ in golden sphere), 2HP–inhibited LOXL2 (LTQ–2HP and three His in cyan, peptide in pale cyan, Cu2+ in slate sphere) and the Zn2+–bound precursor (Tyr687 and three His in green, peptide in pale green, Zn2+ in grey sphere). Right: Overlay of Tyr687, LTQ–2HP and LTQ. (D) Acidic residues (Asp/Glu) and His residues in the active site. In orange stick: conserved acidic residues. In orange line: acidic residues in LOXL2 that are not conserved in the human LOX–family of proteins. In yellow stick: conserved acidic residue as a part of Ca2+–binding site. His623 and His653 (in cyan stick) are conserved. His637 (in blue line) is conserved in human LOX–family of proteins (Figure 4A) but not in mammalian LOXL2 (Figure 4B).