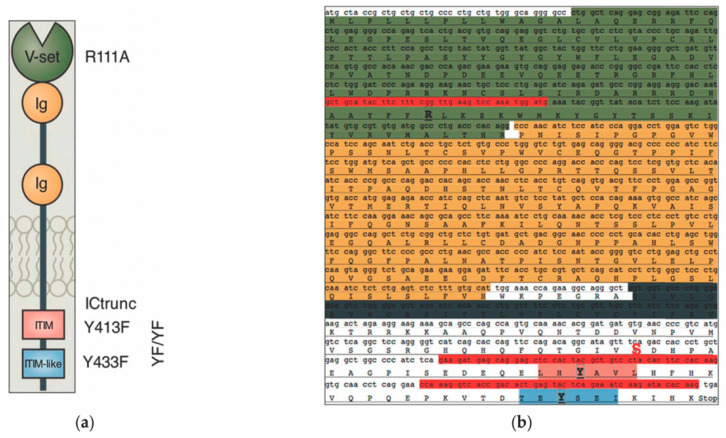
Figure 1.
(a) A graphical representation of Siglec-6 representing the structural components of the proteins including the v-set sialic-acid binding domain (green), two extracellular immunoglobulins (Ig) domains (gold), the transmembrane domain (grey), and the intracellular ITIM (pink) and ITIM-like (blue) putative signaling motifs (adapted from [9]). (b) SIGLEC6 gene sequence with primers used for mutagenesis (highlighted in red) and specific site-directed mutations that were created in the structural domains (highlighted in bold): Alanine to arginine mutation in the v-set domain (R111A), Tyrosine to phenylalanine mutations in the membrane-proximal ITIM (Y413F), distal ITIM-like (Y433F), and both (YFYF). A serine1521 to a STOP codon mutation was created to generate an intracellularly truncated version of Siglec-6 (ICTrunc) (highlighted in red and bold).