Figure 3.
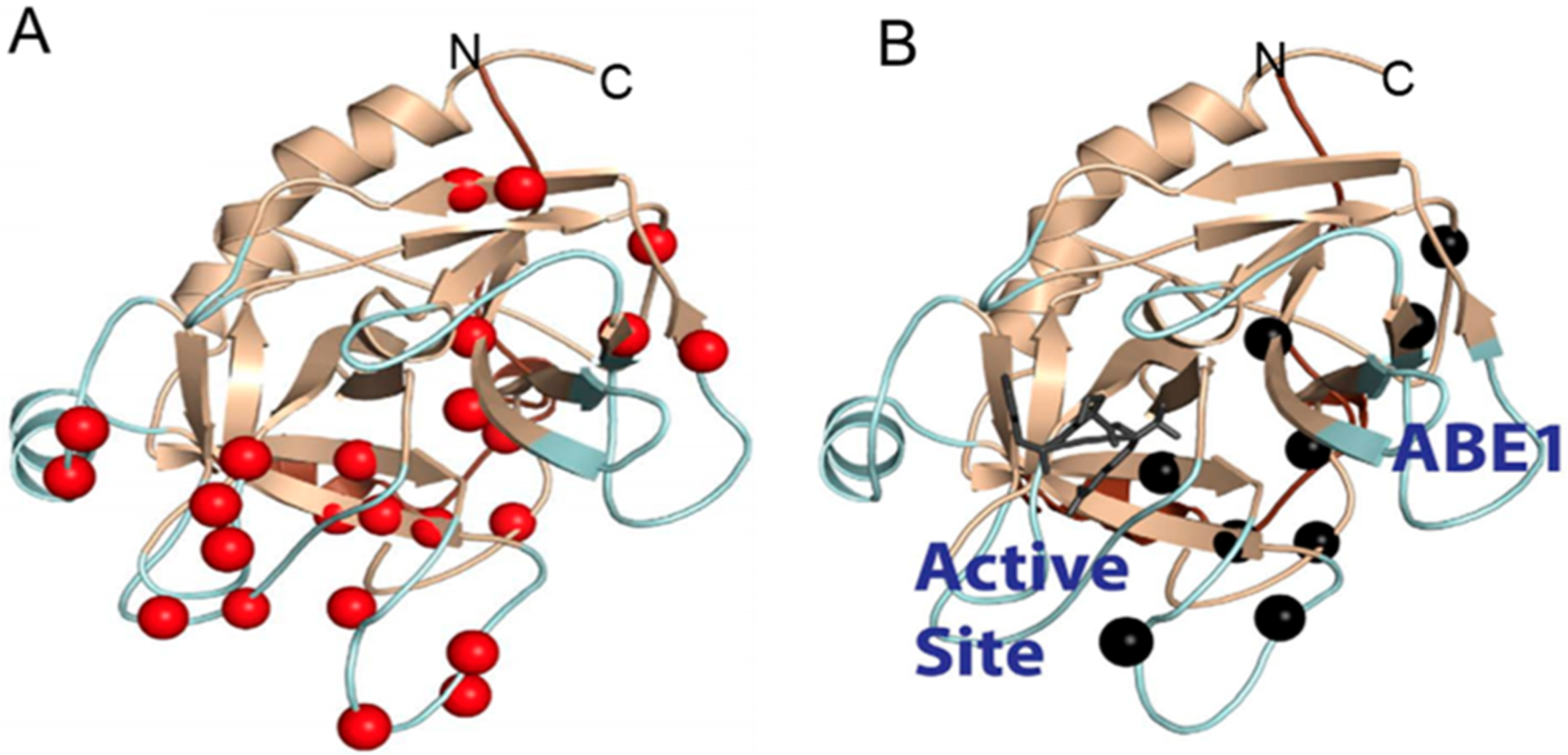
(A) Relaxation dispersion experiments revealed many backbone NH groups throughout the structure of thrombin were moving on the microsecond to millisecond time scale (red spheres). (B) When the covalent active site inhibitor, PPACK, is bound, motions in the C-terminal β-barrel are damped but motions in the N-terminal β-barrel remain and form a pathway of the remaining dynamics from the allosteric site (anion binding exosite 1) to the active site (marked by the PPACK inhibitor in dark gray).
