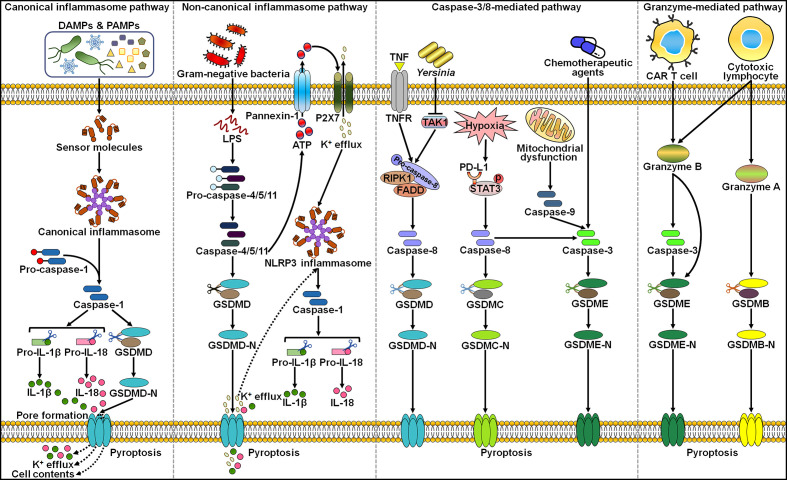
Figure 1.
The main molecular mechanisms of pyroptosis. In the canonical inflammasome pathway, cellular signals (e.g. DAMPs and PAMPs) can activate the canonical inflammasome and capase-1. Active caspase-1 cleaves GSDMD to unleash its N-terminal domain (GSDMD-N), which perforates the cell membrane by forming oligomeric pores. These events lead to the loss of membrane integrity and cell lysis. In addition, caspase-1 cleaves pro-IL-1β and pro-IL-18 into their mature forms, which are released into the extarcellular milieu through the GSDMD-N pore. In the non-canoncial inflammasome pathway, cytosolic LPS from invading Gram-negative bacteria directly activates inflammatory caspase-4/5/11, thus evoking GSDMD-mediated pyroptosis. The GSDMD-N pore causes the efflux of potassium (K+), eventually inducing the assembly of NLRP3 inflammasome and caspase-1-dependent IL-1β and IL-18 secretion. In addition, acitve caspase-11 induces the opening of the pannexin-1 channel, and thus tiggers the extracellular release of ATP, which activates NLRP3 inflammasome via the P2X7 receptor. In caspase-8-mediated pathway, the pathogenic Yersinia infection results in inhibition of the TAK1 activity, motivating the RIPK1/caspase-8 pathway. Active caspase-8 cleaves GSDMD to produce GSDMD-N, resulting in pyroptotic cell death. Under hypoxia conditions, p-STAT3 physically binds to PD-L1 and favors its nuclear translocation, where PD-L1 enhances GSDMC gene expression. TNF-α-activated caspase-8 specifically shears GSDMC, liberating its N-terminal domain (GSDMC-N) that punches holes in the cell membrane and triggers pyroptosis. In capase-3-mediated pathway, chemotherapeutic agent-activated caspase-3 converts GSDME into GSDME-N, which perforates the cell membrane to initiate pyroptosis. In the granzyme-mediated pathway, CAR T cells prompt caspase-3/GSDME-dependent pyroptosis in target cells by secreting granzyme B. Futhermore, granzyme B directly cuts GSDME to drive pyroptosis. In addition, cytotoxic lymphocyte-derived granzyme A can activate the pyroptosis signaling pathway via specific cleavage of GSDMB. DAMPs, damage-associated molecular patterns; PAMPs, pathogen-associated molecular patterns; IL-1β, interleukin-1β; IL-18, interleukin-18; GSDMD, gasdermin D; GSDMD-N, the N-terminal domain of gasdermin D; LPS, lipopolysaccharide; ATP, adenosine triphosphate; P2X7, purinergic receptor P2X ligand-gated ion channel 7; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor; RIPK1, receptor-interacting protein kinase 1; FADD, Fas-associated protein with death domain; TAK1, transforming growth factor-β (TGF-β)-activated kinase 1; PD-L1, programmed death-ligand 1; STAT3, signal transducer and activator of transcription 3; GSDMC, gasdermin C; GSDMC-N, the N-terminal domain of gasdermin C; GSDME, gasdermin E; GSDME-N, the N-terminal domain of gasdermin E; CAR, chimeric antigen receptor; GSDMB, gasdermin B; GSDMB-N, the N-terminal domain of gasdermin B.