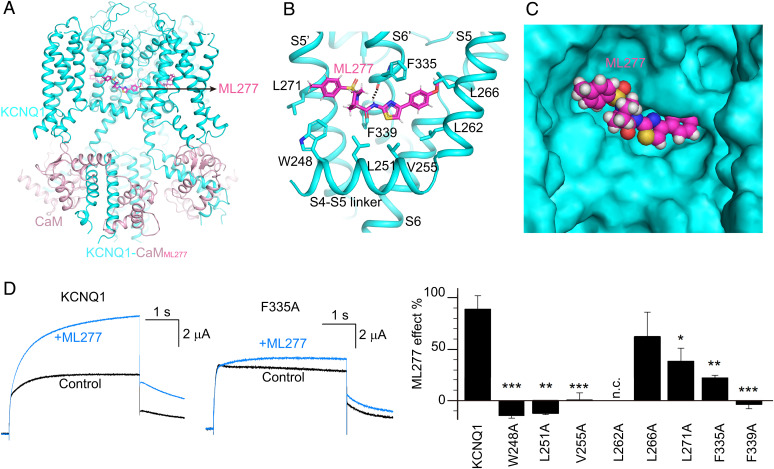
Fig. 4.
The ML277 binding mode in KCNQ1. (A) The overall structure of KCNQ1-CaMML277. KCNQ1, CaM, and ML277 are colored in cyan, pink, and magenta, respectively. (B) The binding site of ML277 in KCNQ1. Side chains of residues in KCNQ1 involved in the hydrophobic interactions with ML277 are shown as sticks. The dashed line indicates the hydrogen bond between ML277 and Phe335. (C) The binding pocket of ML277 in KCNQ1. KCNQ1 is rendered as a surface model, and ML277 is shown as spheres. (D) Left, representative currents of WT KCNQ1 and the F335A mutant before (black) and after (blue) adding 1 µM ML277. Currents were recorded at +40 mV for 4 s and then back to −40 mV to induce the tail currents. Right, averaged results of 1 µM ML277-induced current increase on WT KCNQ1 and its mutations. L262A showed no current (n.c.). n ≥ 4. The P values are 0.00017 for W248A, 0.0011 for L251A, 0.00013 for V255A, 0.30 for L266A, 0.048 for L271A, 0.0016 for F335A, and 0.00091 for F339A.