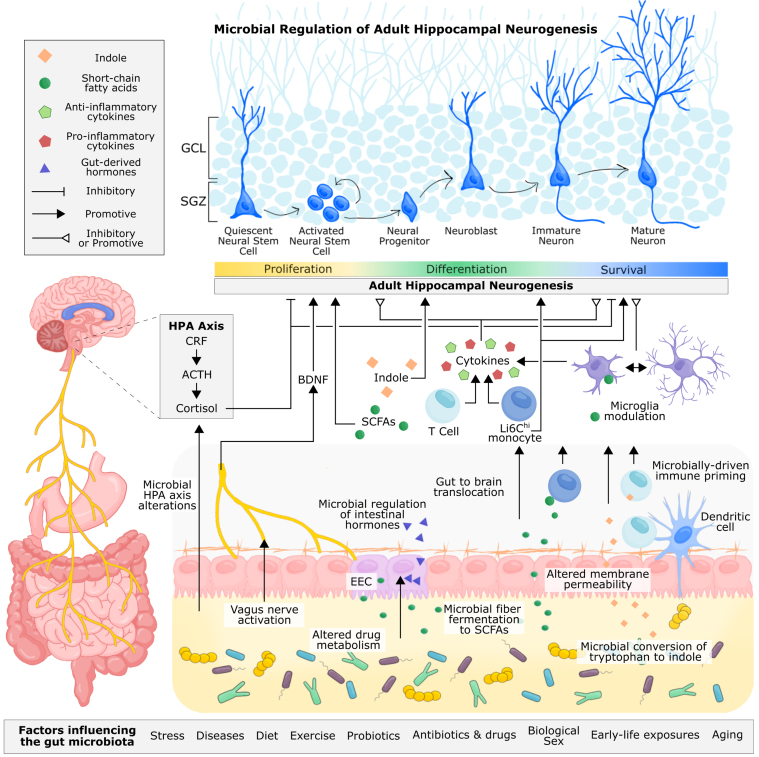
Fig. 4.
Proposed and established mechanisms of microbial regulation of adult hippocampal neurogenesis. Microbe-derived signals can be transmitted to the brain through neuronal, endocrine, metabolite-driven, and immune pathways. The vagus nerve allows for direct neuronal transmission of gut signals to the nucleus tractus solitarius in the brain, which can be relayed to the hippocampus via the medial septum. Through fecal microbiota transplant and probiotic supplementation, microbial signalling from the gut has been shown to alter levels of hippocampal BDNF and influence adult hippocampal neurogenesis. Immune cells circulating the gut, including monocytes and T cells, are sensitive to the gut microbiota, reacting to microbial cell wall components, such as lippopolysaccharide, and microbially-produced metabolites, such as short-chain fatty acids and indoles, that permeate the intestinal epithelium. These immune cells are also primed by the gut microbiota during early life, shaping their functionality in adulthood. T cells and monocytes can traffic to the brain, where they, along with resident microglia, can produce pro-inflammatory and anti-inflammatory cytokines, which have been shown to inhibit or promote hippocampal neurogenesis. Microglia are also sensitive to microbially-produced short-chain fatty acids, which can cross the intestinal epithelium and blood-brain barrier to directly modulate the functionality of microglia. In addition to cytokine production, microglia are responsible for pruning neuronal dendrites and supporting neuronal development and survival. The gut microbiota can metabolize tryptophan, a precursor to serotonin, kynurenine, and indole. Indole produced by the gut microbiota can directly bind to aryl hydrocarbon receptors (AhR) on neural progenitor cells, promoting neuronal differentiation. Immune cells are also sensitive to indoles through AhR receptors. Intestinal neurotransmitter and hormone production is mediated by enteroendocrine cells and under partial control by the gut microbiota, but whether microbial regulation of these metabolites directly influences adult hippocampal neurogenesis in vivo is unconfirmed. Studies involving germ-free mice and microbiota transplantation have demonstrated the importance of the gut microbiota for regulating the HPA axis, which contributes to the production of cortisol (or corticosterone in rodents). Glucocorticoids can directly bind to receptors on progenitor cells and immature neurons and generally have a supressive effect on hippocampal neuron proliferation and survival of newborn neurons. In addition to adult hippocampal neurogenesis, the gut microbiota contributes to neuroplasticity, long term potentiation, and intestinal barrier and blood brain barrier permiability, which offers some mechanistic insight into how the gut microbiota may regulate adult hippocampal neurogenesis and related behaviour. Abbreviations: Adrenocorticotropic hormone (ACTH); brain-derived neurotrophic factor (BDNF); orticotropin-releasing factor (CRF); enteroendocrine cell (EEC); hypothalamus-pituitary-adrenal (HPA).