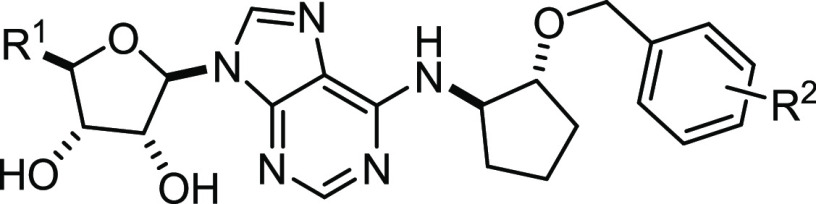
Table 1. Affinity (pKi) and Potency (pEC50) of Extended BnOCPA Derivatives at Human A1Ra.
| compd | R1 | R2 | pEC50 (hA1R)b | Emaxc | pKi (hA1R)d |
|---|---|---|---|---|---|
| BnOCPA | –CH2OH | H | 8.43 ± 0.09 | 51.49 ± 1.9 | 6.18 ± 0.09 |
| 15 | –CH2OH | p-i-Pr | 7.87 ± 0.16 | 51.95 ± 3.1 | 5.77 ± 0.08 |
| 16 | –CH2OH | p-t-Bu | 8.20 ± 0.13 | 54.59 ± 2.8 | 5.58 ± 0.10 |
| 17 | –CH2OH | p-CN | 7.40 ± 0.19 | 58.03 ± 4.2 | 5.85 ± 0.06 |
| 18 | –CH2OH | p-CONH2 | 7.71 ± 0.13 | 59.51 ± 4.1 | 5.98 ± 0.05 |
Data are the mean ± SEM of at least three independent repeats conducted in duplicate.
The negative logarithm of the agonist concentration required to produce a half-maximal inhibition response of the 10 μM forskolin-induced cAMP accumulation in CHO-K1-A1R cells.
The % maximal inhibition of cAMP accumulation for each agonist. Calculated as the % inhibition of the 10 μM forskolin response.
Binding affinity (pKi) determined through the NanoBRET binding assay in HEK293 cells stably expressing human Nluc-A1R. The resulting concentration-dependent decrease in NanoBRET ratio at 10 min was used to calculate pKi. Statistical significance (*p < 0.05) determined using one-way ANOVA and Dunnett’s post-test and presented as described by Curtis et al.31