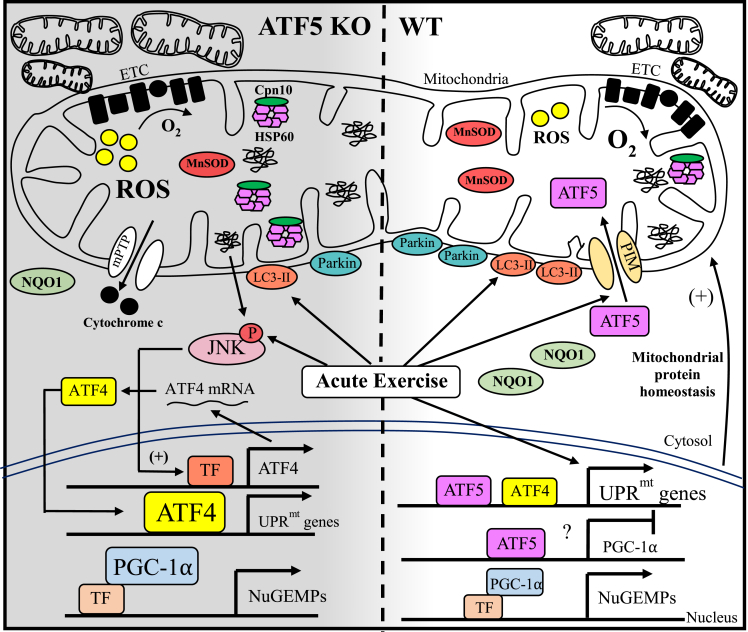
Figure 8.
Working model illustrating the altered muscle physiology of ATF5 KO mice. The absence of ATF5 in skeletal muscle yields a more abundant mitochondrial pool composed of organelles that are less functional. This is characterized by reductions in oxygen consumption and enhanced ROS emission in comparison to WT mitochondria. Increases in ROS may be exacerbated by decreases in the expression of the antioxidant enzymes MnSOD and NQO1, inducing an increase in apoptotic cytochrome c release into the cytosol via the mPTP. The increased abundance of nuclear PGC-1 in the absence of ATF5 may be contributing to the transcription of nuclear genes encoding mitochondrial proteins (NuGEMPs) as well as increases in mitochondrial content observed, suggesting that ATF5 may be a negative regulator of PGC-1 in WT conditions. An enlarged mitochondrial pool in KO muscle may also be attributed to decrements in basal mitophagy indicated by reduced mitochondrial Parkin. ATF5 KO animals also exhibit a blunted mitochondrial quality control response to acute exercise stress, with an attenuated induction of mitochondrial LC3-II and the transcription of UPRmt mRNAs. However, the increased mRNA levels and nuclear localization of ATF4 may explain the enhanced expression of chaperones HSP60 and Cpn10 basally in the muscle of ATF5 KO mice. Despite the attenuated mitochondrial response (above), an increased stress kinase signaling was evident post-exercise in these animals, represented by enhanced JNK phosphorylation. The activation of JNK could also result from mitochondrial proteotoxicity to induce the transcription of ATF4 and the increase in ATF4 expression. Finally, acute exercise appears to induce the import of ATF5 into mitochondria, rather than to the nucleus. Solid arrows indicate evidence that signaling is occurring.