Figure 1.
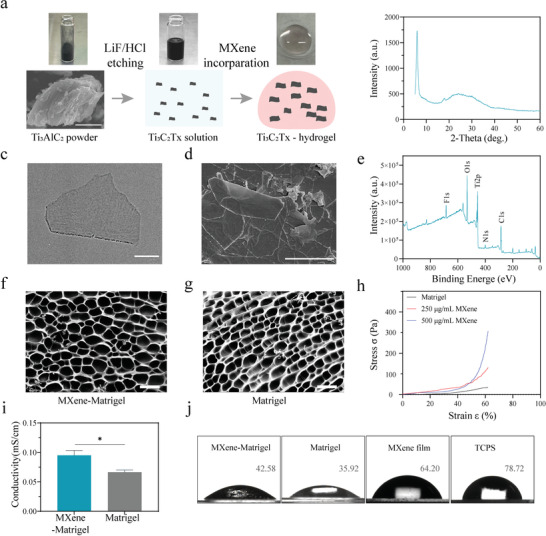
Ti3C2TxMXene and MXene‐Matrigel hydrogel preparation and characterization. a) Schematic diagram of the preparation of Ti3C2TxMXene and MXene‐Matrigel. Appropriate amount of Ti3C2TxMXene solution was mixed with Matrigel, and the incorporating hydrogel was solidified at 37 °C. b) The XRD pattern of Ti3C2TxMXene. The characteristic peak of Ti3C2TxMXene was prior to 8°. c) Representative TEM image of the Ti3C2TxMXene nanosheets. Scale bar = 200 nm. d) SEM image of the Ti3C2TxMXene solution, which was diluted to 100 µg mL−1 by water. Scale bar = 2 µm. e) XPS patterns of the Ti3C2TxMXene film. Abundant functional groups containing O, Ti, and C were detected. f,g) SEM images of MXene‐Matrigel (f) and Matrigel (g). Scale bar = 50 µm. h) Stress‐strain curve of Matrigel and MXene‐Matrigel (250 µg mL−1 Ti3C2TxMXene, red; 500 µg mL−1 Ti3C2TxMXene, blue). i) The conductivity of Matrigel and MXene‐Matrigel (250 µg mL−1). (Data are presented as mean ± SD, n = 3, * p < 0.05). j) The water contact angles of MXene‐Matrigel (250 µg mL−1), Matrigel, Ti3C2TxMXene film, and TCPS.
