Figure 5.
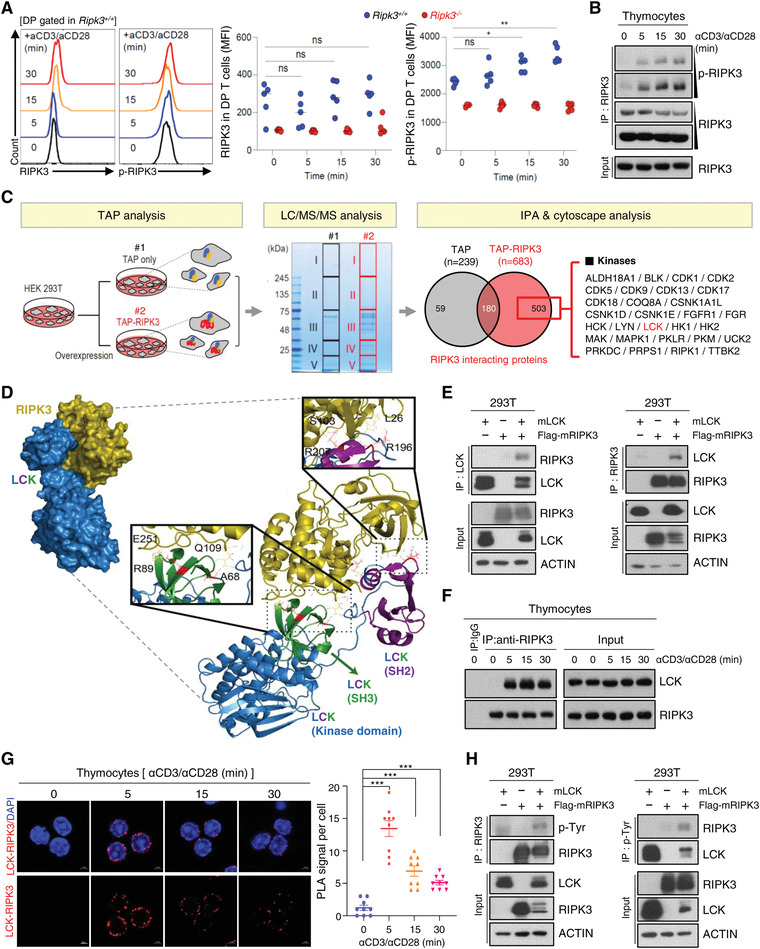
TCR‐mediated activation of LCK interacts with and RIPK3 to promote RIPK3 phosphorylation. A) Total thymocytes from Ripk3+/+ (n = 5) and Ripk3−/− (n = 5) mice were cultured with anti‐CD3 (1 µg mL−1) and anti‐CD28 (2 µg mL−1) or without for indicated time. Relative fluorescence of RIPK3 and p‐RIPK3 in DP T cells. B) Phosphorylation of RIPK3 increased in anti‐CD3 (1 µg mL−1) and anti‐CD28 (2 µg mL−1) stimulation. C) Experimental workflow for TAP (Tandem Affinity purification) pull‐down assay. An aliquot of each purified sample was loaded to SDS‐PAGE and stained with Coomassie brilliant blue. Potential RIPK3‐binding proteins were identified by LC‐MS/MS. Venn diagram represents the overlap of proteins and unique proteins identified by LC/MS/MS among TAP‐purified samples as indicated. Total 503 proteins were identified as specific RIPK3 binding proteins. There were several kinases and phosphatases. D) Computational docking model for RIPK3 (olive) and LCK (blue) predicted using ClusPro (see Materials and Methods). E) Western blot analysis after immunoprecipitation of mouse RIPK3 and mouse LCK in HEK293T cells. HEK293T cells were transfected with LCK and/or Flag‐RIPK3 expression constructs. Cells were harvested at 24 h after transfection. The endogenous RIPK3 interacted with LCK in response to anti‐CD3/CD28 stimulation. Interaction of RIPK3 and LCK was observed by F) Western blot analysis, and confirmed with G) Duolink proximity ligation assay. H) Tyrosine phosphorylation of RIPK3 were detected in LCK and Flag‐RIPK3 expressing HEK293T cells. Statistical analyses were performed using the two‐tailed unpaired Student t‐test. p values below 0.05 were considered significant in the following manner: *p < 0.05, **p < 0.01, ***p < 0.001.
