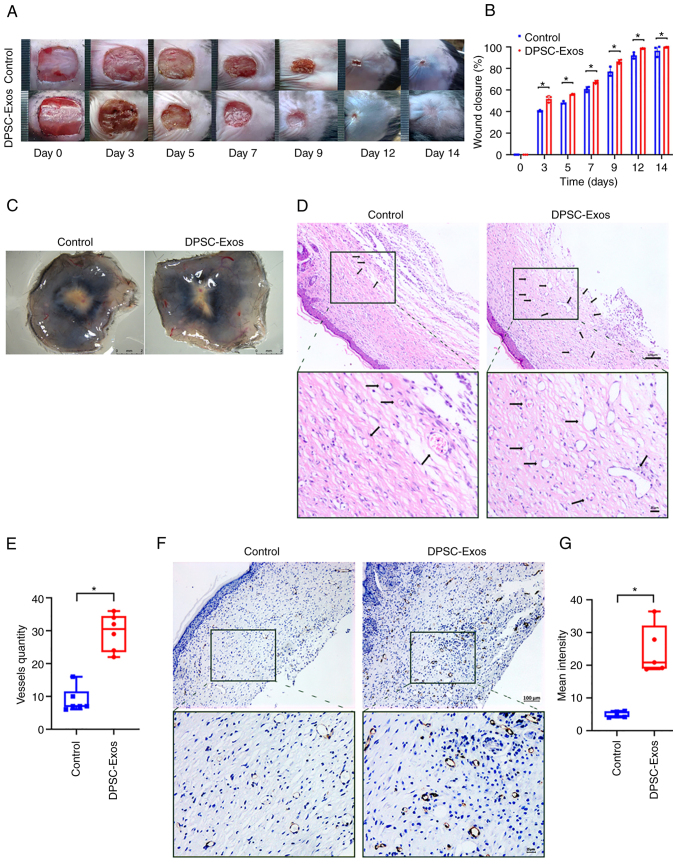
Figure 1.
DPSC-Exos accelerate cutaneous wound healing in mice by promoting angiogenesis. (A) Gross view and quantification of wound area of mice treated with PBS and DPSC-Exos on days 3, 5, 7, 9, 12 and 14 post-wounding. Scale bar, 1 cm. (B) The rate of wound closure in wounds receiving DPSC-Exos treatments was significantly higher at the indicated time points (n=3). (C) Gross view of wounds of mice treated with PBS and DPSC-Exos on day 14 post-wounding from the undersurface. More newly formed blood vessels were detected in the wound sites of the DPSC-Exo-treated group. Scale bar, 2 mm. (D and E) Hematoxylin & eosin staining of the wound sections treated with PBS and DPSC-Exos on day 14 after the operation. The black arrows indicate newly formed blood vessels. The vessel quantity in the DPSC-Exo-treated group was larger than that of the control group (n=3). Scale bar, 100 µm. (F and G) Immunohistochemical staining for CD31 in wound sections of mice treated with PBS and DPSC-Exos at 14 days after the operation. A higher expression of CD31 was found in the DPSC-Exo-treated group (n=3). Scale bar, 100 µm. *P<0.05. DPSC, dental pulp stem cell; Exo, exosome; PBS, phosphate-buffered saline.