FIGURE 1.
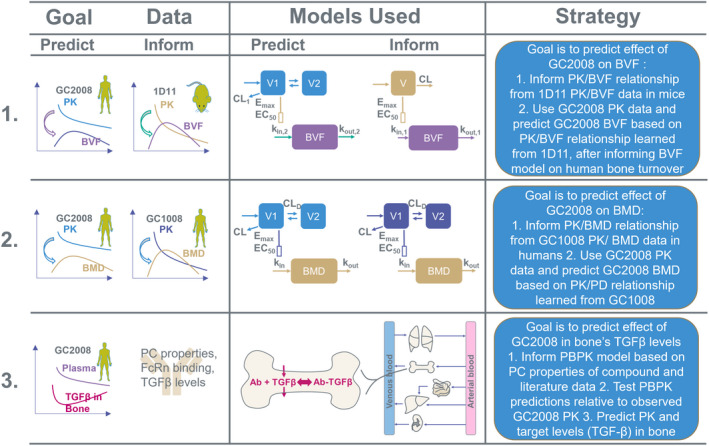
Schematic of the multi‐model strategy followed to predict efficacious human dose of the anti‐TGF‐β antibody (GC2008) for the treatment of OI. In the first modeling approach the goal was to predict efficacious dose based on the effect of GC2008 on bone volume fraction (BVF = bone volume/total volume BV/TV). In this, a PK/PD (BVF) relationship was established from 1D11 PK/BVF data in mice. To predict the response for GC2008 in humans, the PK component of the model was replaced to represent GC2008 PK, and the PD parameters governing the half‐life were replaced to represent human bone turnover rate. The model was then used to predict the dose of GC2008 that will lead to a 5% increase in BVF. In the second modeling approach, the goal was to predict the efficacious dose based on the effect of GC2008 on BMD. In this, a PK/PD (BMD) relationship was established from Fresolimumab (GC1008) PK/BMD data. Next, PK data from GC2008 was used along with the Fresolimumab (GC1008) PD‐related parameters to provide a dose that results in a 5% increase in BMD. In the third modeling approach a PBPK model was informed based on drug's physicochemical (PC) properties, TGF‐β levels, and human physiology. After checking the validity of PBPK model's predictions by comparing with GC2008 PK data, the PBPK model was used to evaluate the dose that will decrease OI‐related TGF‐β levels back to their homeostatic concentration. BMD, bone mineral density; CL, clearance; EC50, half‐maximal effective concentration; E max, maximum effect; OI, osteogenesis imperfecta; PBPK, physiologically‐based pharmacokinetic; PD, pharmacodynamic; PK, pharmacokinetic.
