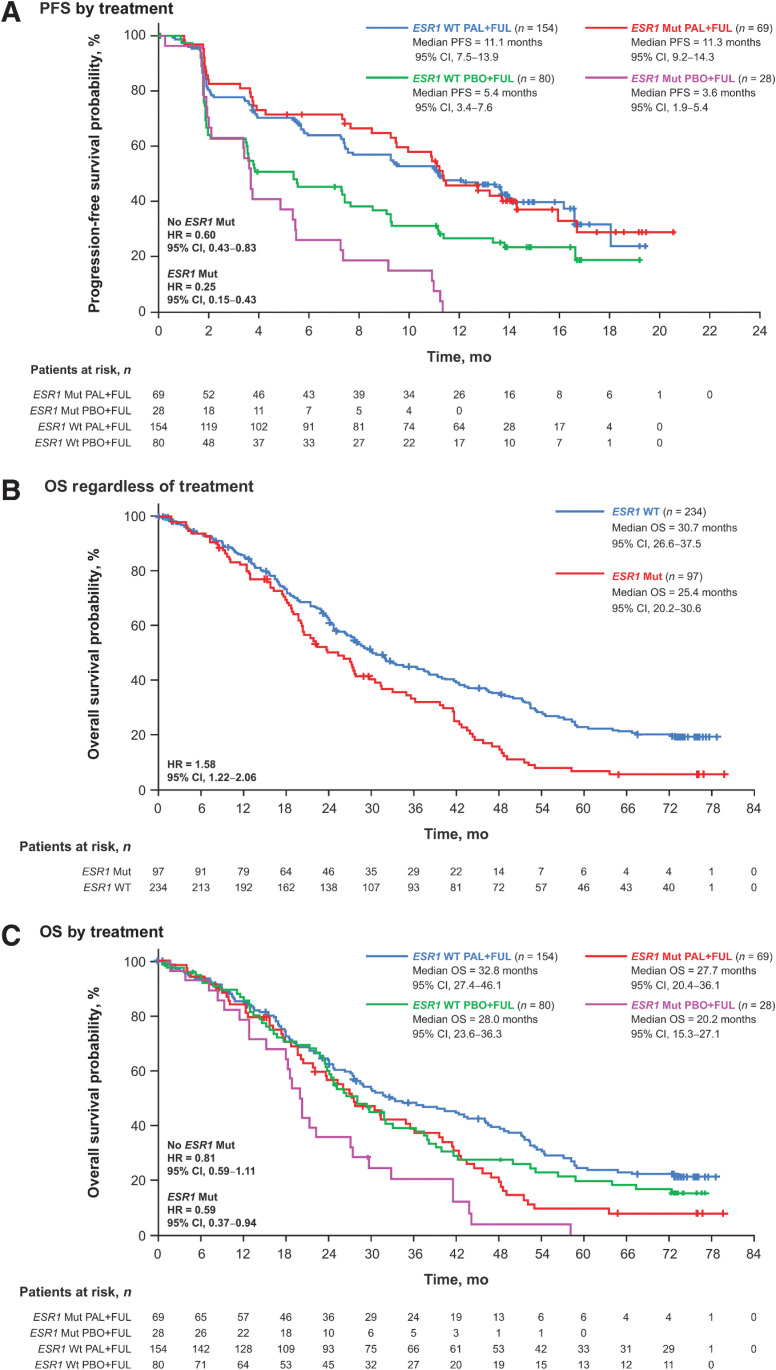
Figure 3.
Outcomes by ESR1 mutation status in ctDNA at day 1 or end of treatment. PFS by treatment (A), OS regardless of treatment (B), and OS by treatment (C). ctDNA, circulating tumor DNA; ESR1, estrogen receptor 1; FUL, fulvestrant; HR, hazard ratio; mut, mutation; OS, overall survival; PAL, palbociclib; PBO, placebo; PFS, progression-free survival; WT, wild type.