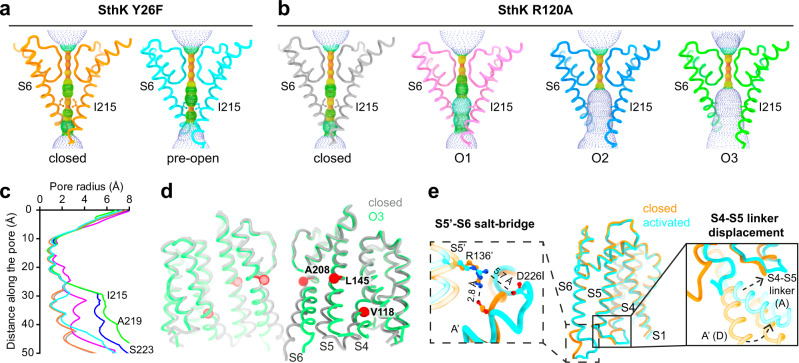
Fig. 3. Gating intermediates display gradually increased pore openings.
a, b Show the pore helices, selectivity filters, and S6 pore lining helices from two opposing subunits (cartoon) and the pore radius (spheres, calculated using HOLE) for SthK Y26F in (a) and SthK R120A in (b). Ile215, which forms the main constriction, is highlighted and shown as sticks. c Plot of the calculated pore radii for all proteins used in this study with colors as in (a, b). d Overlay of SthK R120A closed (gray) and O3 (green) states showing displacements of S4, S5, S6 during channel opening. Hinge points on each helix, where movements begin, are highlighted (red) and the corresponding residues are labeled. e TMD of one subunit of SthK Y26F (closed in orange, pre-open in cyan) is shown. Zoom-ins focus on the Arg136-Asp226 inter-subunit salt-bridge holding the bundle crossing closed (left) and the S4-S5 linker displacement in the pre-open state allowing the C-linker to move upwards closer to the membrane.