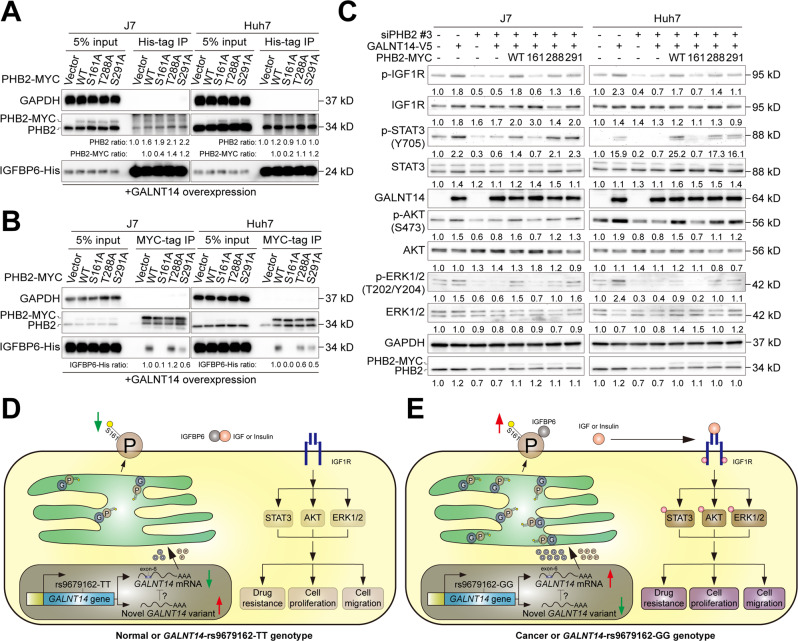
Fig. 7. GALNT14-mediated O-glycosylation of PHB2 at Ser161 promotes IGF1R-mediated signaling through the association of PHB2 with IGFBP6.
A, B Western blot analysis of co-immunoprecipitation assays using HCC cell samples treated as indicated. C Western blot analysis of HCC cell lysates treated as indicated. D, E Working model of this study. The newly discovered short RNA variant of GALNT14 is present and able to attenuate the expression of exon-6-containing mRNA in normal hepatocytes or in patients carrying the rs9679162-TT genotype. Consequently, lower levels of GALNT14, PHB2, and O-glycosylated PHB2 are produced, preventing activation of IGF1R-mediated signaling (D). However, in cancerous hepatocytes or patients carrying the rs9679162-GG genotype, the novel RNA variant of GALNT14 was absent and failed to suppress the expression of mRNA containing exon-6, resulting in increased levels of GALNT14, PHB2, and O-glycosylated PHB2. The increase in O-glycosylated PHB2 competes for binding with IGFBP6, thereby increasing IGF (or insulin) release to activate the IGF1R-mediated signaling cascade.