Abstract
Surgical treatment of superior sulcus tumors (SSTs) is clinically challenging. Definitive chemoradiotherapy (CRT) is a standard treatment for SST. In operable cases, multimodal therapy (CRT followed by surgery) is another option, at least for experienced institutions. Immune checkpoint inhibitors (ICIs) have recently been developed, and several clinical trials have investigated definitive CRT followed by ICIs for consolidation or maintenance therapy of unresectable local advanced non‐small cell lung cancer (NSCLC), including SSTs. Clinical studies of salvage surgery after CRT followed by ICIs are also ongoing. However, the clinical outcomes of salvage surgery after multimodal therapies and histopathological analyses of surgical specimens after such treatments remain unclear. Here, we report the case of a patient with SST comprising squamous cell carcinoma with invasion of the second to third rib and vertebrae who underwent salvage surgery after concurrent definitive CRT followed by the ICI durvalumab, and show the results of clinicopathological analyses of the resected specimen.
Keywords: immune checkpoint inhibitors, lung cancer, salvage surgery, superior sulcus tumor
Multimodal therapies including ICIs may provide a promising option to improve surgical resectability with negative surgical margins for clinically challenging cases of local advanced lung cancer, including superior sulcus tumors.
INTRODUCTION
Surgical treatment of superior sulcus tumors (SSTs) is clinically challenging. Definitive chemoradiotherapy (CRT) is a standard treatment for SST. In operable cases, multimodal therapy (CRT followed by surgery) is another option, at least for experienced institutions. Immune checkpoint inhibitors (ICIs) have recently been developed, and several clinical trials have investigated definitive CRT followed by ICIs for consolidation or maintenance therapy of unresectable local advanced non‐small cell lung cance (NSCLCs), including SSTs. Clinical studies of salvage surgery after CRT followed by ICIs are also ongoing. However, the clinical outcomes of salvage surgery after multimodal therapies and histopathological analyses of surgical specimens after such treatments remain unclear. Here, we report the case of a patient with SST comprising squamous cell carcinoma with invasion of the second to third ribs and vertebrae who underwent salvage surgery after concurrent definitive CRT followed by the ICI durvalumab, and show the results of clinicopathological analyses of the resected specimen.
CASE REPORT
A 70‐year‐old man with chronic obstructive pulmonary disease (COPD) as a comorbidity was hospitalized in a nearby hospital with a 2‐month history of back pain and right shoulder pain. Computed tomography (CT) of the chest revealed superior sulcus tumors (SSTs) at the right lung apex with invasion of the second to third ribs and vertebrae (Figure 1a). Subsequent 18F‐fluorodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET) revealed high uptake by the main tumor and in the invaded areas (second to third ribs and vertebrae), but no other distant or lymph node metastases. The patient was diagnosed with squamous cell carcinoma of the right upper lobe (cT4N0M0 IIIA) by CT‐guided biopsy. En bloc curative surgical resection was considered difficult by the surgical teams at the referring hospital. Definitive chemoradiotherapy (CRT) (chemotherapy plus concurrent radiotherapy) was started as cisplatin and vinorelbine at two cycles, and 60 Gy in 30 fractions. After CRT, the immune checkpoint inhibitor (ICI) durvalumab was administered as consolidation therapy every 2 weeks up to 12 months. Follow‐up CT revealed partial response of the main tumor, and complete response of the area of vertebral invasion (second to third rib vertebral invasion) after treatments (Figure 1b). However, regrowth of the main tumor was identified 1 year after starting the ICI without any growth in the invaded vertebral area (Figure 1c). Further 18F‐FDG PET showed uptake in the main tumor with no accumulation in the invaded vertebral area (Figure 1d).
FIGURE 1.
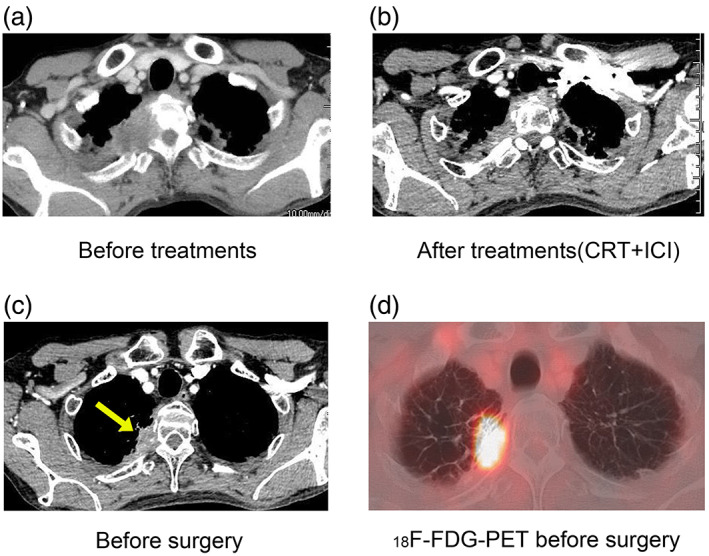
(a) Computed tomography (CT) before treatment shows the right lung cancer invading the second to third rib and vertebrae. (b) CT after multimodal treatments (CRT + ICI) reveals partial response (PD) of the main tumor. (c) Preoperative CT scan shows the regrowth of main tumor. The yellow arrow indicates the main tumor. (d) 18F‐FDG‐ PET scan shows high uptake of regrowth of the main tumor, but no uptake in the vertebrae
The patient was therefore referred to our hospital for salvage surgery. We performed salvage surgery for the residual tumor showing uptake on PET. The operation was started with a transmanubrial approach (TMA) and anterior approach through the fourth intercostal space (using video‐assisted thoracic surgery). Right upper lobectomy and combined resection of the partial second to third ribs and vertebrae were performed, showing negative surgical margins on intraoperative frozen section. The postoperative course was uneventful and the patient experienced no complications. Histopathological analysis showed major pathological response (MPR) with a small number of viable cells in the main tumor and negative surgical margins with no viable cells in the resected ribs and vertebrae (Figure 2a,b). Immunohistochemical staining showed tumor‐infiltrating lymphocytes were positive for PD‐1 (Figure 2c; arrowheads), while there remained no viable cancer cells with positive expression of programmed cell death 1 ligand‐1 (PD‐L1) (Figure 2d; tumor proportion score [TPS] = 0%). A follow‐up CT scan was performed every 6 months after surgery. Two years postoperatively, no recurrences have been seen.
FIGURE 2.
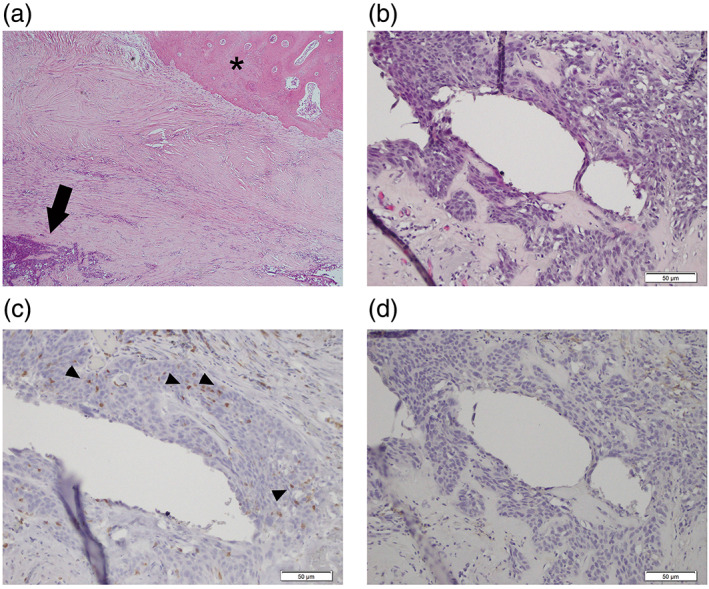
Immunohistochemical staining of surgical specimens. (a) Hematoxylin and eosin (HE) staining shows the major pathological response (MPR). The arrow indicates viable cancer cells. No viable cancer cells existed around the combined resected rib (asterisk). (b) HE staining shows viable cancer cells. (c) Immunohistochemical staining for programmed cell death 1 (PD‐1). Arrowheads indicate tumor‐infiltrating lymphocytes with positive PD‐1 staining. (d) Immunohistochemical staining for programmed cell death 1 ligand‐1 (PD‐L1)
DISCUSSION
Here, we report a clinically successful case in which salvage surgery was performed for an SST involving definitive CRT followed by durvalumab. Surgical resection of SSTs remains clinically challenging, and various surgical approaches have been developed. Difficulty is seen in selecting the appropriate approach due to the anatomical and surgical complexity of the region (tumor location and combined resection involving surrounding structures of great vessels, ribs, and vertebrae), and the indications are not well defined. When a tumor involves anterior structures such as the brachiocephalic vein and subclavian artery, anterior approaches such as a hemi‐clamshell approach or TMA are selected. When the tumor has invaded posterior structures such as the vertebrae or chest walls, a posterolateral approach or hook approach is selected. 1 In the present case, the patient had good local control without any accumulation of 18FDG‐PET in the invaded vertebrae, and only partial vertebrectomy was performed using the anterior approach. To overcome such an advanced local lung cancer including SSTs, one of the most important prognostic factors is complete resection with negative surgical margins for the invaded areas. To date, the main surgical treatment for SST has been preoperative concurrent CRT followed by surgery to achieve complete resection with negative surgical margins (R0). 2 Among several ICIs that have recently been developed, durvalumab (an anti‐PD‐L1 antibody) was administered after CRT in this case as consolidation therapy. Salvage surgery after durvalumab and a clinical trial of neoadjuvant ICIs have been reported. 3 In addition, several clinical studies of induction therapy including ICIs are ongoing. 4 The present case showed good local control with MPR of not only the main tumor, but also the invaded vertebral area. Further, no viable cancer cells were identified in the surgical margins. Multimodal therapies including ICIs may provide a promising option to improve surgical resectability with negative surgical margins for clinically challenging cases of local advanced lung cancer, including SSTs.
In our previous study using in vitro experiments, PD‐L1 expression in lung cancer cells was upregulated after chemotherapy. 5 In addition, histopathological analysis using surgical samples before and after CRT showed that the PD‐L1 TPS after CRT was significantly higher as compared to before CRT. 5 In the histopathological analysis of the present case, some viable cancer cells remained in the main tumor and all the viable cells showed negative PD‐L1 expression, while around the tumor cells, the presence of tumor‐infiltrating lymphocyte (TIL) with positive PD‐1expression were seen. These results suggest that no PD‐L1‐positive cancer cells remained in the residual tumor because PD‐L1‐positive cancer cells upregulated by CRT may be killed by ICIs. However, despite the multimodal therapies including ICIs, cancer cells survived and may have acquired chemo‐, radio‐, and ICI‐resistant characteristics and metastatic or recurrent potential in the future. As the next step, cancer cells surviving multimodal therapies need to be analyzed using molecular biological approaches to achieve a deeper understanding of malignant characteristics.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Funaki S, Ose N, Kimura T, Kanou T, Fukui E, Shintani Y. Clinicopathological analysis of a superior sulcus tumor treated by salvage surgery after concurrent definitive chemoradiotherapy followed by durvalumab: A case report. Thorac Cancer. 2022;13(22):3229–3232. 10.1111/1759-7714.14681
REFERENCES
- 1. Rusch VW, Parekh KR, Leon L, Venkatraman E, Bains MS, Downey RJ, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg. 2000;119(6):1147–53. 10.1067/mtc.2000.106089 [DOI] [PubMed] [Google Scholar]
- 2. Kunitoh H, Kato H, Tsuboi M, Shibata T, Asamura H, Ichinose Y, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non‐small‐cell lung cancers: report of Japan clinical oncology group trial 9806. J Clin Oncol. 2008;26:644–9. [DOI] [PubMed] [Google Scholar]
- 3. Ueno T, Yamashita M, Yamashita N, Uomoto M, Kawamata O, Sano Y, et al. Safety of salvage lung resection after immunotherapy for unresectable non‐small cell lung cancer. Gen Thorac Cardiovasc Surg. 2022;70:812–7. 10.1007/s11748-022-01798-3 [DOI] [PubMed] [Google Scholar]
- 4. Aokage K, Tsuboi M, Zenke Y, Horinouchi H, Nakamura N, Ishikura S, et al. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non‐small cell lung cancer. Jpn J Clin Oncol. 2022;52(4):383–7. 10.1093/jjco/hyab208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Funaki S, Shintani Y, Kawamura T, Kanzaki R, Minami M, Okumura M. Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF‐β induced epithelial mesenchymal transition in non‐small cell lung cancer. Oncol Rep. 2017;38(4):2277–84. 10.3892/or.2017.5894 [DOI] [PubMed] [Google Scholar]


