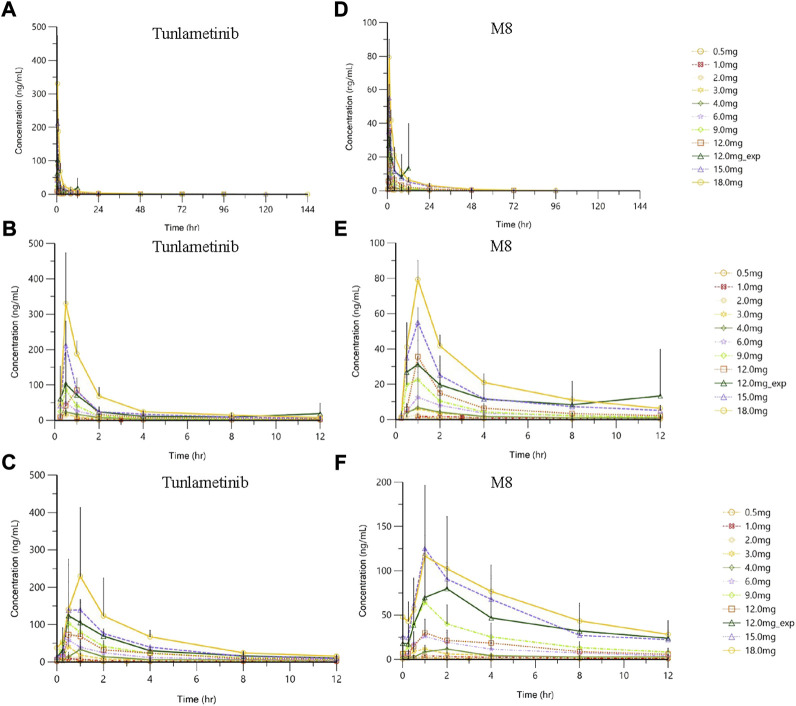
FIGURE 1.
| Plasma concentration (mean ± SD)-time profiles of tunlametinib and M8 after orally administrated with 0.5–18 mg (n = 41) tunlametinib capsules. (A) single dose (0–144 h, tunlametinib); (B) single dose (0–12 h, tunlametinib); (C) multiple dose (tunlametinib); (D) single dose (0–144 h, M8); (E) single dose (0–12 h, M8); (F) multiple dose (M8).