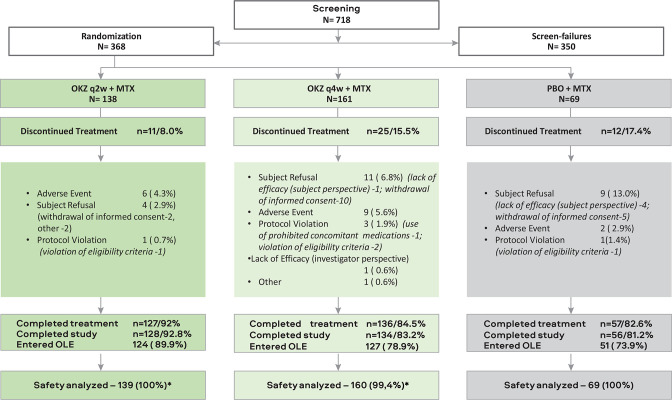
Figure 2.
Patient disposition. *One patient was randomised to OKZ 64 mg q4w but actually received OKZ 64 mg q2w. Patients who discontinued treatment early and entered safety follow-up period were considered completers for the whole study if they performed all three follow-up visits. Therefore, the number of those who completed study can be higher than the number of treatment completers. AE, adverse event; IC, informed consent; ITT, intention-to-treat; MTX, methotrexate; N, number patient in the arm; N (%), number (%) patients; %, the percentage of subjects is calculated relative to the total number of subjects in the population; OKZ, olokizumab; OLE, open-label extension; PBO, placebo; q2w, every 2 weeks; q4w, every 4 weeks.