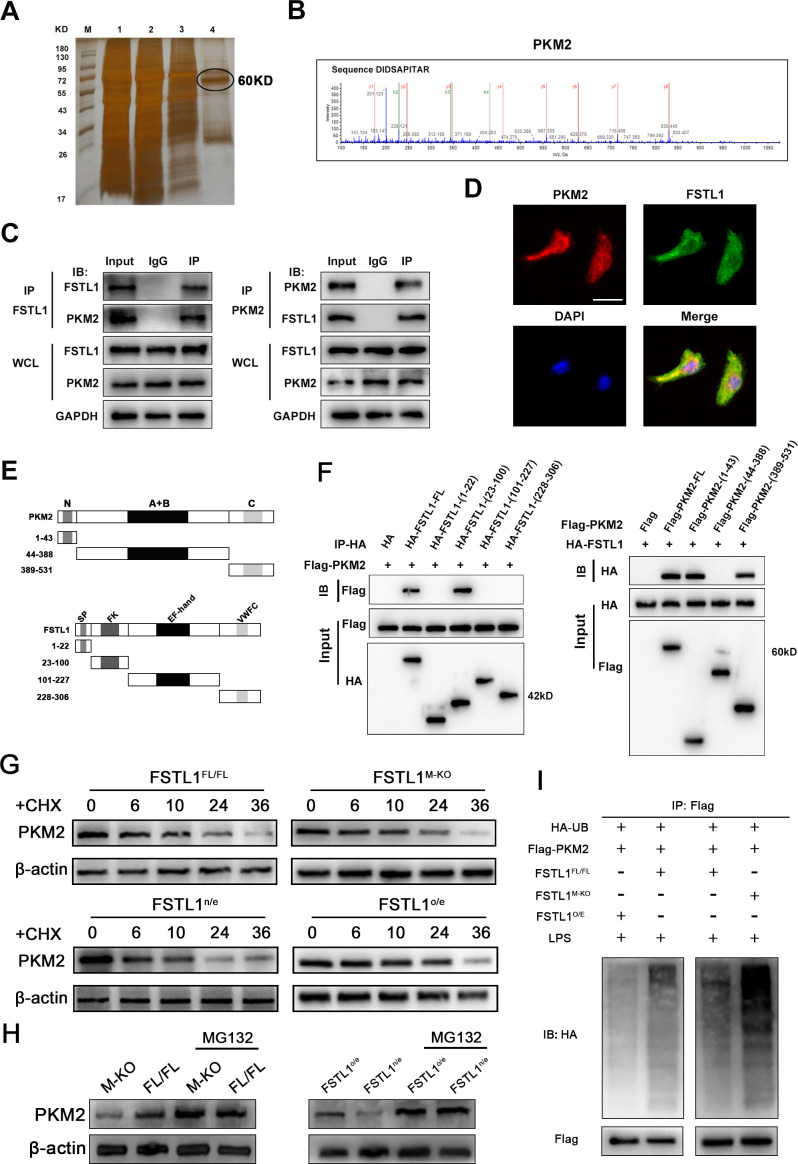
Figure 5.
Follistatin-like protein 1 (FSTL1) directly binds with pyruvate kinase M2 (PKM2) and enhances the stability of PKM2 in macrophages. (A) The Co-immunoprecipitation (Co-IP) complex was subjected to silver-staining (line 4 represented the eluted protein); (B) the peptide sequences of the PKM2 protein as detected in the Co-IP complex by mass spectrometry; (C) western blot (WB) analysis of Co-IP complex confirmed that FSTL1 interacted with PKM2 in bone marrow-derived macrophages (BMDMs); (D) the immunofluorescence showed that FSTL1 colocalised with PKM2 and were expressed in both the cytoplasm and nucleus of BMDMs; (E) the structural compositions of FSTL1 and PKM2; (F) WB analysis of relationship between truncated FSTL1 and full-length PKM2 or truncated PKM2 and full-length FSTL1; (G) BMDMs extracted from FSTL1FL/FL and FSTL1M-KO mice and BMDMs extracted from FSTL1FL/FL transfected with LV-FSTL1 or lentivirus-negative control (LV-NC) were treated with cycloheximide (100 ng/mL) for the indicated periods of time; (H) BMDMs extracted from FSTL1FL/FL and FSTL1M-KO mice and BMDMs extracted from FSTL1FL/FL transfected with LV-FSTL1 or LV-NC were treated with or without MG132 (50 µM) for 6 hours. (I) In vitro ubiquitination assay of FSTL1FL/FL, FSTL1M-KO and FSTL1o/e BMDMs. All cells were co-transfected with FLAG-PKM2 and HA-UB plasmid. Cell lysates were immunoprecipitated with anti-FLAG antibody followed by immunoblotting analysis with anti-HA or anti-FLAG antibody. Scale bars, 50 µm.