Abstract
Hyperammonaemia syndrome secondary to Ureaplasma spp. infection is well documented in the post-lung transplant population. We report a case of a man in his fifties with hyperammonaemia syndrome secondary to disseminated Ureaplasma parvum infection. This occurred in the context of immunosuppression for chronic graft versus host disease and six years following an allogeneic stem cell transplant for diffuse large B-cell lymphoma. Following treatment of U. parvum septic arthritis with ciprofloxacin and doxycycline, the patient experienced a full neurological recovery, and continues on suppressive doxycycline therapy with no recurrence of symptoms to date.
Keywords: Malignant disease and immunosuppression, Bone and joint infections, Infectious diseases
Background
Organisms of the genus Ureaplasma belong to the bacterial class ‘mollicutes’ and the two main species are Ureaplasma urealyticum and Ureaplasma parvum.1 Ureaplasma spp. colonise the mucosal surfaces of the cervix and vagina in females and the lower urethra in males to a lesser degree.1 It is well established that these organisms are implicated in urogenital infections and are associated with adverse pregnancy outcomes in women. Invasive Ureaplasma spp. infections outside of this paradigm are less common and tend to predominate in patients with humoral immunodeficiency, either congenital or acquired.2
Hyperammonaemia syndrome (HS) following lung transplant is a well-known complication in the early transplant period,3 and the link to Ureaplasma spp. infection has been recently established.4–6
A review of HS secondary to disseminated Ureaplasma spp. infection outside the lung transplant population prior to January 2022 identified only eight other published case reports. All cases were diagnosed using molecular methods and most cases were treated with empirical dual-antimicrobial therapy, presumably due to the lack of culture and susceptibility testing to guide directed therapy. We report a case of disseminated U. parvum infection with HS following allogeneic stem cell transplant who was successfully treated with ciprofloxacin and doxycycline combination therapy.
Case presentation
A man in his fifties presented to the emergency department (ED) with confusion, fevers and a painful, hot, swollen native left knee joint. He was significantly agitated, requiring chemical sedation. He had an elevated white cell count (WCC) (15.10×109/L), C reactive protein (311 mg/L) and procalcitonin (0.55 ng/mL). The left knee was aspirated, demonstrating an elevated WCC (23.4 ×109/L) and nil organisms or crystals on microscopy. He was commenced on empiric antibiotics for septic arthritis with intravenous flucloxacillin and vancomycin.
Prior to admission, he was a full-time working professional and had a good functional and cognitive baseline. His medical history was significant for diffuse large B-cell lymphoma, stage 2a at diagnosis seven years prior to presentation. He was treated with induction chemotherapy (prednisone, methotrexate, cyclophosphamide) and salvage vincristine/gemcitabine with partial response. He proceeded to allogeneic stem-cell transplant from a matched sibling donor with reduced-intensity conditioning within a year of his original diagnosis. This was complicated by acute and then chronic graft versus host disease (GVHD) with cutaneous, ocular and hepatic involvement. He had trialled multiple lines of therapy and at the time of admission was receiving imatinib, ruxolitinib and prednisone 15 mg. He also suffered hypogammaglobulinaemia, due to poor uptake of his graft, for which he received fortnightly subcutaneous immunoglobulin.
The patient underwent operative left knee joint washout which demonstrated gross synovitis and turbid fluid throughout the joint consistent with septic arthritis. However, there was no growth on bacterial cultures from both preoperative and intraoperative samples. Postoperatively, he was treated with intravenous cephazolin and vancomycin but continued to spike fevers. Antibiotic therapy was changed to oral clindamycin and ciprofloxacin, with subsequent resolution of fevers. He was discharged home and completed a six-week course of clindamycin and ciprofloxacin.
One-week postcessation of antibiotics, the patient represented to ED with confusion and a painful, hot, swollen native right knee joint (i.e. contralateral to the initial presentation). He was again febrile and agitated requiring chemical sedation with droperidol. His right knee aspirate revealed an elevated WCC (12.3×10ˆ9/L), no organisms or crystals on microscopy and he proceeded to an operative right knee joint washout. He was commenced on intravenous piperacillin/tazobactam postoperatively.
In recovery, he was reviewed for a decreased Glasgow Coma Score (GCS) of 3. He was normoglycaemic (blood glucose level 9 mmol/L) but hypertensive (systolic blood pressure 200 mm Hg) requiring intravenous hydralazine. Arterial blood gas revealed respiratory alkalosis (pH 7.53, paCO2 31 mm Hg, paO2 99 mm Hg) and lactate 1.8 mmol/L. Computed tomography (CT) of the brain excluded intracranial haemorrhage. He was reintubated and admitted to the intensive care unit. At the time, there was concern regarding the possible prolonged effect of droperidol causing drowsiness and the intensivist planned to monitor lactate, electrolytes, glucose, ammonia, liver functions and perform an electroencephalogram the following morning.
The patient’s serum ammonia level returned significantly elevated at 269 umol/L (upper limit of normal: 50 umol/L). The procalcitonin was elevated at 4.15 ng/mL.
Differential diagnosis
The patient’s decreased GCS was attributed to hyperammonaemic encephalopathy. In the absence of liver failure, non-hepatic causes of hyperammonaemia were considered, including infections due to urease-producing organisms. Joint fluid was tested for Mycoplasma spp. and Ureaplasma spp. by polymerase chain reaction (PCR) and 16s ribosomal ribonucleic acid (rRNA) sequencing. Doxycycline 100 mg two times per day was added to his antimicrobial regimen while results were pending.
There was no growth on bacterial, fungal and mycobacterial cultures from the right knee joint aspirate and intraoperative right knee joint fluid. U. parvum nucleic acid was detected in right knee joint fluid by PCR and 16 s rRNA sequencing.
Treatment
He was treated with benzoic acid (to decrease glyceine metabolism), lactulose (to decrease gut absorption of ammonia) and continuous renal replacement therapy in an attempt to reduce blood ammonia levels. When U. parvum was detected in joint fluid, his antimicrobial regimen was changed to a combination of ciprofloxacin 400 mg two times per day intravenously and doxycycline 100 mg two times per day via nasogastric tube to treat the underlying cause of hyperammonaemia.
Outcome and follow-up
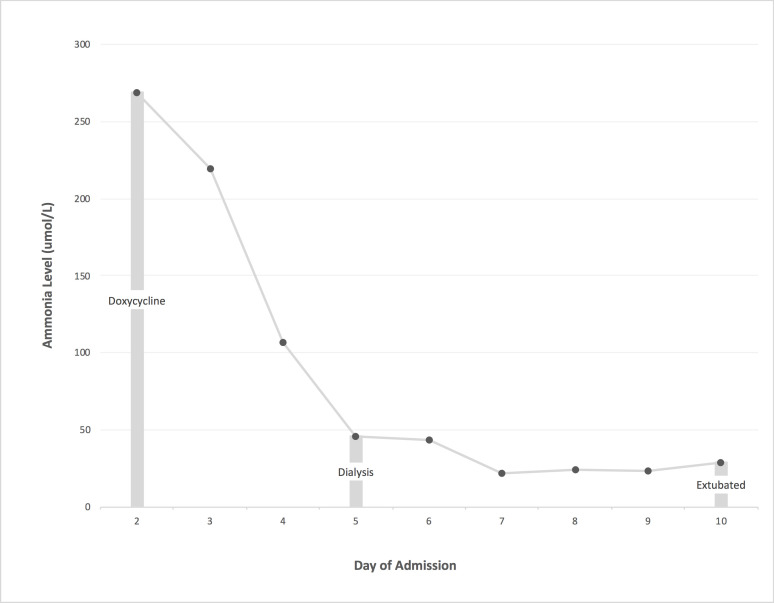
The patient’s serum ammonia level normalised with treatment (see figure 1) and he had a corresponding full neurological recovery. The patient completed two weeks of intravenous ciprofloxacin and continues on oral doxycycline 100 mg two times per day with plans to continue suppressive therapy lifelong (or until immunologlobulin levels return to normal). He has not had a recurrence of symptoms more than a year following completion of acute treatment.
Figure 1.
Serum ammonia levels with intervention during second admission.
Discussion
The diagnosis of Ureaplasma spp. infections is difficult for a number of reasons. Firstly, Ureaplasma spp. are unable to be visualised by Gram stain because they lack a peptidoglycan cell wall.7 Secondly, they are difficult to grow using standard bacterial culture methods and require complex growth media.7 As such, diagnosis often relies on molecular methods such as PCR and 16s rRNA sequencing,3 as we saw in this case.
Antimicrobial treatment options for Ureaplasma infections are limited. Agents that target the bacterial cell wall, such as beta lactams, have no activity against these organisms given they lack a peptidoglycan cell wall.1 Furthermore, Ureaplasma spp. do not produce folic acid and as such sulphonamides and trimethoprim are ineffective.1 Tetracyclines, macrolides (protein synthesis inhibitors) and quinolones (nucleic acid synthesis inhibitor) all have theoretical activity against Ureaplasma spp.1 Studies suggest that most Ureaplasma spp. in the United States of America are susceptible to tetracyclines, while up to 68.8% of isolates are resistant to ciprofloxacin.7 Given the organism is not readily cultured in routine practice, treatment is largely empirical.
Hyperammonaemia is caused by either increased ammonia production or decreased ammonia elimination. The most common cause of hyperammonaemia in adults is fulminant liver failure resulting in decreased ammonia elimination.8 Other causes of decreased ammonia elimination include portosystemic shunting, drugs disrupting the urea cycle (especially in patients with urea cycle disorders) and in-born errors of metabolism. Infections with urease-producing bacteria such as Ureaplasma spp. result in increased ammonia production. Increased ammonia production can also occur as a result of gastrointestinal haemorrhage, total parenteral nutrition, trauma/burns and multiple myeloma.8
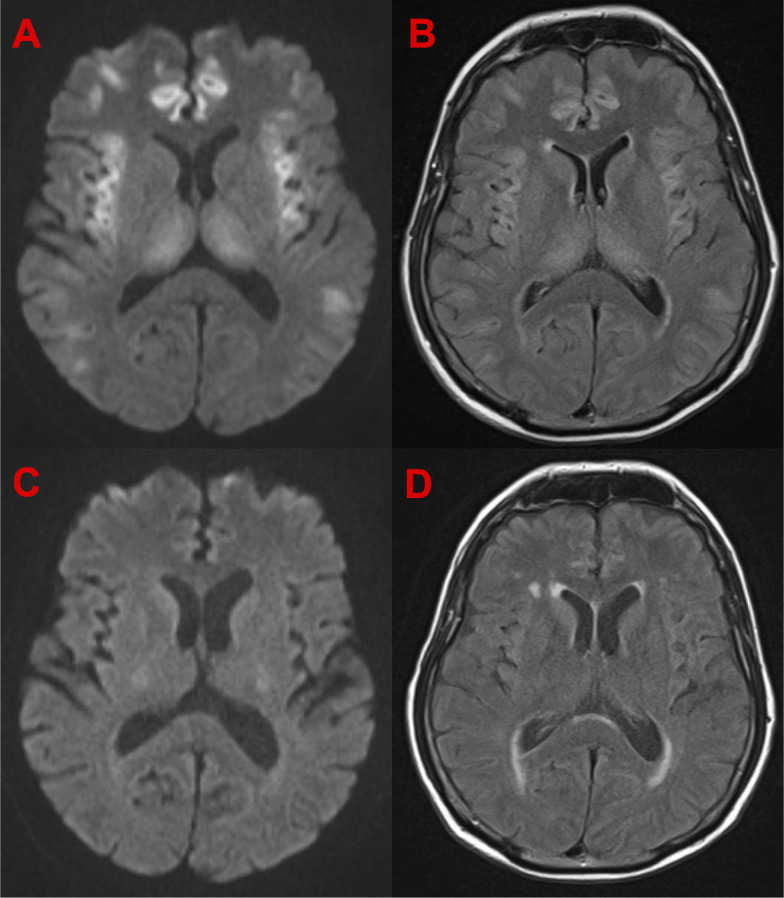
HS is characterised by elevated ammonia levels in the absence of synthetic liver dysfunction, with altered mental state (eg, agitation, decreased GCS) and cerebral oedema.3 On neuroimaging, extensive cortical injury and a finding of bilateral and symmetrical involvement of the insular and cingulate cortices is suggestive of hyperammonaemic encephalopathy.9 The cortical changes have been shown to be largely reversible, which is consistent with what was seen in our case (see figure 2).
Figure 2.
Axial diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) MRI sequences of the patient during hyperammonaemia and with normalisation of the serum ammonia levels). (A) DWI MRI sequences showing diffusion restriction during hyperammonaemia. (B) FLAIR MRI sequences showing hyperintensities during hyperammonaemia. (C) DWI MRI sequences with normalisation of serum ammonia showing resolution of areas of diffusion restriction. (D) FLAIR MRI sequences with normalisation of serum ammonia showing resolution of areas hyperintensity.
HS secondary to Mycoplasma hominis and Ureaplasma spp. has been described in immunocompromised hosts. U. urealyticum and U. parvum produce large amounts of urease which hydrolyses urea to produce adenosine triphosphate (ATP), generating ammonia in the process.3
HS following lung transplant is a rare but well established complication in the early transplant period.3 Given the relative infrequency of this condition, the link between HS and infection with Ureaplasma spp in this cohort has only been recently established. A longitudinal study published in 2015 4 identified systemic Ureaplasma spp. infection via PCR in six post-lung transplant patients with HS and none of the twenty control subjects. U. parvum and U. urealyticum were subsequently shown to cause hyperammonaemia in an immunosuppressed murine model.5 6 Following these findings, HS secondary to Ureaplasma spp. has been reported outside the lung transplant population.
A literature search using PubMed and MEDLINE databases and the search terms “Ureaplasma” and “Hyperammonemia” was conducted. We found only eight other cases of HS secondary to ureaplasma infection, outside the lung transplant population, published in English (see table 1).
Table 1.
Previously reported cases of hyperammonaemia syndrome secondary to Ureaplasma spp infection outside the lung transplant population
| Case/(reference) | Year published | Age | Sex | Risk factor | Site of infection | Species | Diagnostic test | Treatment | Outcome |
| 1/10 | 2020 | 16 | F | Newly diagnosed acute myeloid leukaemia undergoing induction chemotherapy | Primary site not identified | U. parvum | PCR on blood | Doxycycline | Alive |
| 2/11 | 2019 | 32 | F | Acute lymphoblastic leukaemia undergoing salvage chemotherapy | Primary site not identified | U. urealyticum | PCR on blood | Levofloxacin | Died |
| 3/12 | 2018 | 21 | M | Post stem cell transplant for acute myeloid leukaemia | Pneumonia | U. parvum | PCR on tracheal aspirate | Azithromycin+levofloxacin | Alive |
| 4/13 | 2021 | 53 | F | Chimeric receptor antigen T-cell recipient for relapsed acute lymphoblastic leukaemia | Pneumonia | Not specified | PCR on BAL fluid | Levofloxacin | Died |
| 5/14 | 2020 | 16 | F | Kidney transplant | Joint (polyarthritis) | U. urealyticum | PCR on blood, urine, synovial fluid | Doxycycline+levofloxacin | Alive |
| 6/15 | 2020 | 53 | F | Liver-kidney transplant | Endovascular infection+Peritonitis | Not specified | PCR on intra-abdominal collections and stent | Doxycycline+levofloxacin | Alive |
| 7/16 | 2020 | 56 | F | Kidney transplant | Urinary tract | Not specified | 16s on urine | Moxifloxacin+doxycycline | Alive |
| 8/17 | 2020 | 65 | F | Kidney transplant | Surgical site infection “chronic scar infection post kidney transplant” | U. parvum | PCR on blood and urine | Doxycycline+levofloxacin | Died |
BAL, Bronchoalveolar lavage; F, female; M, male.
Half of the cases (4/8) occurred following solid-organ transplants (kidney and kidney/liver). The remaining cases (4/8) occurred in patients with haematological malignancies either currently undergoing chemotherapy10 11 or within 2 weeks of haematopoietic stem-cell transplant (HSCT)12 or chimeric antigen receptor T-cell therapy.13 This is in contrast to our case where HS occurred more than 5 years post-HSCT. Notwithstanding, our case did have other significant risk factors including hypogammaglobulinaemia and being on immunosuppressive treatment for chronic GVHD. More than half of the cases (62.5% (5/8)) were treated with dual antimicrobial therapy. The most common regimen was a combination of doxycycline and quinolone, as was used in our case. In 37.5% (3/8) of cases, HS had a fatal outcome, of which two out of the three cases were treated with levofloxacin monotherapy.
Learning points.
The most common cause of hyperammonaemia is fulminant liver failure. In the absence of liver dysfunction, consider extrahepatic causes such as infection with urease-producing organisms.
Diagnosis of infections caused by Ureaplasma spp is difficult because routine bacterial culture methods will not culture the organism. Request specific molecular testing if there is an index of suspicion.
Treatment of Ureaplasma spp infections is chiefly empirical. Options include tetracyclines, macrolides and quinolones.
Footnotes
Contributors: NB, KO, MJM and SH were involved in the patient’s care and contributed to writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev 2005;18:757–89. 10.1128/CMR.18.4.757-789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jhaveri VV, Lasalvia MT. Invasive Ureaplasma Infection in Patients Receiving Rituximab and Other Humoral Immunodeficiencies-A Case Report and Review of the Literature. Open Forum Infect Dis 2019;6:ofz399. 10.1093/ofid/ofz399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leger RF, Silverman MS, Hauck ES, et al. Hyperammonemia post lung transplantation: a review. Clin Med Insights Circ Respir Pulm Med 2020;14:117954842096623. 10.1177/1179548420966234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharat A, Cunningham SA, Scott Budinger GR, et al. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med 2015;7:284re3. 10.1126/scitranslmed.aaa8419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Karau MJ, Greenwood-Quaintance KE, et al. Ureaplasma urealyticum causes hyperammonemia in an experimental immunocompromised murine model. PLoS One 2016;11:e0161214. 10.1371/journal.pone.0161214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Greenwood-Quaintance KE, Karau MJ, et al. Ureaplasma parvum causes hyperammonemia in a pharmacologically immunocompromised murine model. Eur J Clin Microbiol Infect Dis 2017;36:517–22. 10.1007/s10096-016-2827-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández J, Karau MJ, Cunningham SA, et al. Antimicrobial susceptibility and clonality of clinical Ureaplasma isolates in the United States. Antimicrob Agents Chemother 2016;60:4793–8. 10.1128/AAC.00671-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest 2007;132:1368–78. 10.1378/chest.06-2940 [DOI] [PubMed] [Google Scholar]
- 9.U-King-Im JM, Yu E, Bartlett E, et al. Acute hyperammonemic encephalopathy in adults: imaging findings. AJNR Am J Neuroradiol 2011;32:413–8. 10.3174/ajnr.A2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Placone N, Kao RL, Kempert P, et al. Hyperammonemia from Ureaplasma infection in an immunocompromised child. J Pediatr Hematol Oncol 2020;42:e114–6. 10.1097/MPH.0000000000001414 [DOI] [PubMed] [Google Scholar]
- 11.Nowbakht C, Edwards AR, Rodriguez-Buritica DF, et al. Two Cases of Fatal Hyperammonemia Syndrome due to Mycoplasma hominis and Ureaplasma urealyticum in Immunocompromised Patients Outside Lung Transplant Recipients. Open Forum Infect Dis 2019;6:ofz033. 10.1093/ofid/ofz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graetz R, Meyer R, Shehab K, et al. Successful resolution of hyperammonemia following hematopoietic cell transplantation with directed treatment of Ureaplasma parvum infection. Transpl Infect Dis 2018;20:e12839. 10.1111/tid.12839 [DOI] [PubMed] [Google Scholar]
- 13.Tawfik P, Arndt P. Lethal hyperammonemia in a CAR-T cell recipient due to Ureaplasma pneumonia: a case report of a unique severe complication. BMJ Case Rep 2021;14:e242513. 10.1136/bcr-2021-242513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins AB, Farmakiotis D, Rogers R, et al. Hyperammonemia syndrome due to Ureaplasma urealyticum in a kidney transplant recipient: a case of disseminated disease from a fluoroquinolone-resistant isolate. Transpl Infect Dis 2020;22:e13328. 10.1111/tid.13328 [DOI] [PubMed] [Google Scholar]
- 15.Cannon CA, Corcorran MA, Shaw KW, et al. Hyperammonemia syndrome due to Ureaplasma infection after liver-kidney transplant. Transpl Infect Dis 2020;22:e13298. 10.1111/tid.13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheema F, Kutzler HL, Olowofela AS, et al. Successful management of noncirrhotic hyperammonemia syndrome after kidney transplantation from putative Ureaplasma infection. Transpl Infect Dis 2020;22:e13332. 10.1111/tid.13332 [DOI] [PubMed] [Google Scholar]
- 17.Legouy C, Hu A, Mochel F, et al. Ureaplasma parvum causes hyperammonemia presenting as refractory status epilepticus after kidney transplant. J Crit Care 2020;57:79–83. 10.1016/j.jcrc.2020.02.003 [DOI] [PubMed] [Google Scholar]