Table 6.
Identification of eluting chemicals from Tampax Cup after one boiling cycle followed by wiping with two Feminine Always Wipes.
| No. | Identification | CAS Number | Molecular formula | Structure | Confidence a |
|---|---|---|---|---|---|
| 1 | Valine | 516-06-3 | C5H11O2N |  |
Matched |
| 2 | EDTA | 60-00-4 | C10H16O8N2 | 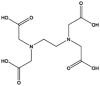 |
Matched |
| 3 | Sugar-like constituents | NA | C6H12O5 C12H22O9 C10H18O9 – C50H82O42 |
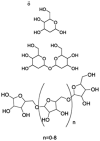 |
Tentative |
| 4 | Citric acid | 77-92-9 | C6H8O7 |  |
Matched |
| 5 | PEG 40 Hydrogenated Castor Oil | 61788-85-0 | Mixture | Mixture | Reference |
| 6 | Sorbitan caprylate | 60177-36-8 | C14H26O6 |  |
Reference |
| 7 | Isosorbide monocaprylate | 49553-31-3 | C14H24O5 | 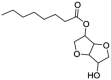 |
Reference |
| 8 | Dioctanoate sorbitan | 152261-28-4 | C22H40O7 |  |
Reference |
| 9 | 12-hydroxystearic acid | 106-14-9 | C18H36O3 | Tentative | |
| 10 | Diethylene glycol mono (12-hydroxystearate) | 122105-30-0 | C22H44O5 | Reference | |
| 11 | Trioctanoate sorbitan | 94131-37-0 | C22H44O5 |  |
Reference |
| 12 | 11-Carboxy-1-hexylundecyl 12-hydroxystearate | 218291-10-2 | C36H70O5 | Reference | |
| 13 | Triethylene glycol di (12-hydroxystearate) | 124615-57-2 | C42H82O8 | Reference |
Abbreviations: EDTA, Ethylene diamine tetra-acetic acid; PEG, Polyethylene glycol.
Colour legend: Structure components highlighted in green indicate the connectivity of the two moieties, often at one of the hydroxy groups. However, the exact hydroxy group cannot be determined without a standard.
Level of confidence classifications: (i) A partial identification denotes the determination of one or more functional groups OR a proposed molecular formula. (ii) A tentative identification is a proposed structure derived by using commonly accepted mass spectrometry interpretation practices AND a secondary piece of mass spectral data, published material composition, or chromatographic information that supports the proposed chemical identification. (iii) A matched compound identification is valid when a proposed compound chemical identification can be justified by one of the following: (a) using a published or user library spectral match AND the identification can be deemed as reasonable given additional knowledge of the sourcing or composition of the sample; (b) OR a molecular formula derived from using accurate mass AND the product ion spectrum is consistent with the proposed identification AND the identification can be deemed as reasonable given additional knowledge of the sourcing or composition of the sample. (iv) A reference identification occurs when the requirements of Matched identification are met AND there exists a mass spectral and retention time match to a reference standard containing the purported material.
