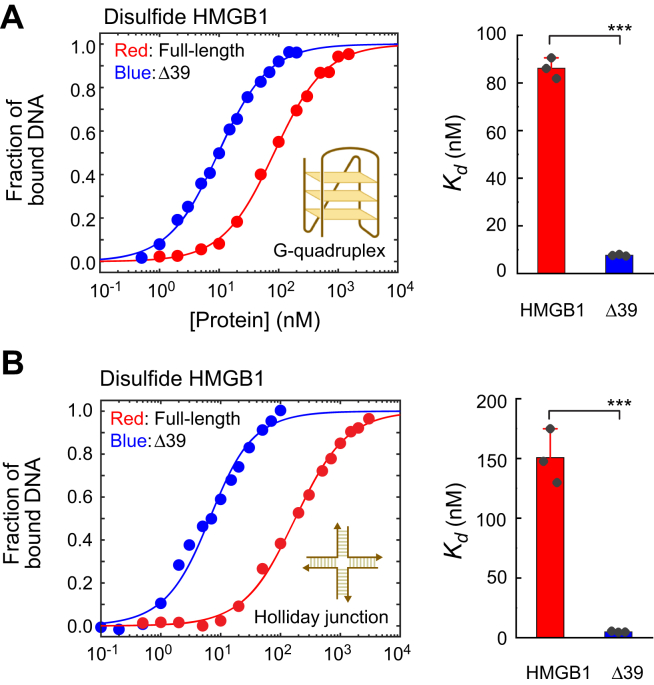
Figure 3.
The main product of HMGB1 proteolysis by neutrophil elastase exhibits substantially stronger binding affinity for DNA G-quadruplex and Holliday junction. Fluorescence anisotropy-based binding assays were used to measure affinities for a G-quadruplex and a Holliday junction. A, binding isotherm data for the interactions of the intact disulfide HMGB1 and Δ39 proteins with FAM-labeled G-quadruplex DNA (32-mer). Corresponding data for the Δ40 and Δ44 products are shown in Fig. S5 in the Supporting information. B, binding isotherm data for the interactions of the intact disulfide HMGB1 and Δ39 proteins with FAM-labeled Holliday junction. For each Kd, error bars represent the SDs for three replicates. ∗∗∗p < 0.001.