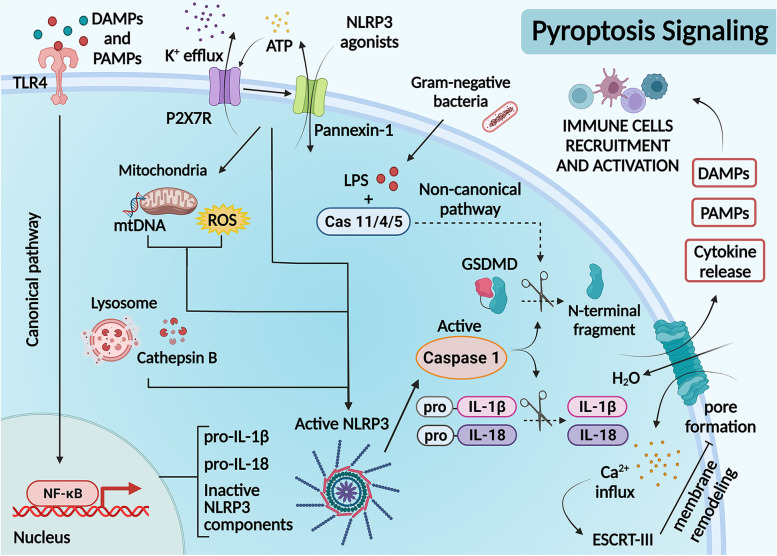
Fig. 1.
Mechanisms of canonical and non-canonical pyroptotic cell death. Canonical pyroptotic cell death activation is triggered in response to various stimuli. The first signal (priming step) is initiated through the identification of PAMPs and DAMPs by the pattern recognition receptors (PRRs) including toll-like receptors (TLRs). This first signal activates the NF-κB transcription factor, which encodes for pro-IL-1β, IL-18, and inactive NLRP3 components. Subsequently, other agonists act as the second signal (activation step), for example; extracellular ATP sensing that activates P2 × 7 receptors, leading to potassium efflux and activating Pannexin-1, allowing intracellular ATP release. These alterations can trigger an accumulation of mitochondrial reactive oxygen species (ROS), mitochondrial membrane permeabilization, and cathepsin B release by damaged lysosomal compartments. These signals stimulate NLRP3 inflammasome assembly and activation, inducing caspase-1 self-cleavage, resulting in IL-1β and IL-18 maturation and subsequent cleavage of Gasdermin D (GSMD). The N-terminal GSDMD domain forms membrane pores, releasing intracellular components, such as cytokines, PAMPs, and DAMPs, resulting in the recruitment of immune cells and their activation. Conversely, non-canonical activation requires direct binding of caspase-4/5 (in humans) or caspase-11 (in mice) to cytosolic lipopolysaccharides. Active caspase then cleaves GSDMD leading to pyroptosis. A variety of molecules can be modulated to induce or inhibit any step of this signaling cascade. As an example, ESCRT-III is modulated upon certain conditions as an attempt to remodel the membrane pores and diminish both pyroptosis and cytokine secretion, being an attractive target for regulating pyroptosis in different models