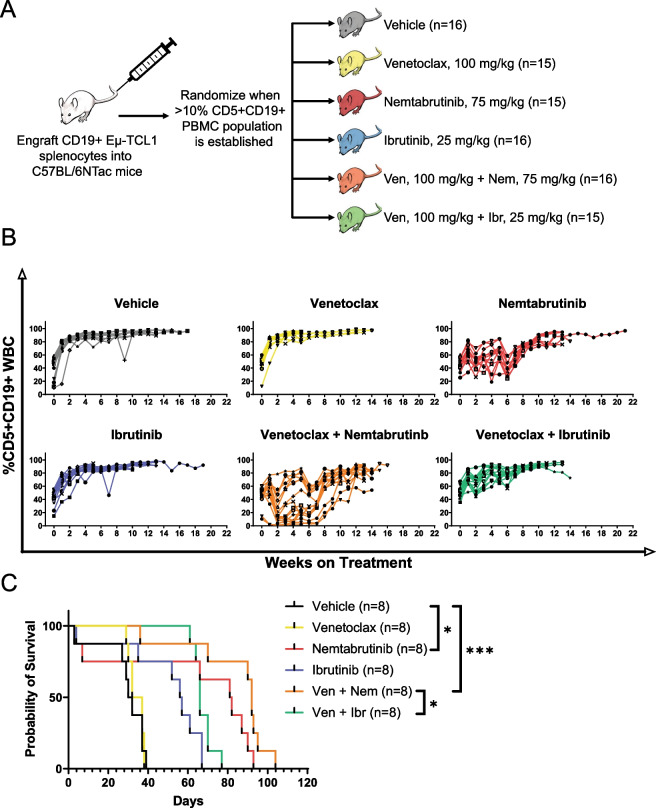
Fig. 2.
A Splenocytes from 4 Eμ-TCL1 donors were engrafted into C57BL/6NTac mice (n = 93) via tail vein injection in groups of 48, 25, 17, and 3 recipient mice, respectively. Recipient mice were randomly enrolled into treatment groups after developing CD5 + CD19 + disease as assessed by flow cytometry of peripheral blood. Mice were treated daily via oral gavage with vehicle, venetoclax (100 mg/kg), nemtabrutinib (75 mg/kg), ibrutinib (25 mg/kg), venetoclax (100 mg/kg) and nemtabrutinib (75 mg/kg), or venetoclax (100 mg/kg) and ibrutinib (25 mg/kg). Mice receiving single agents were administered two gavages: one gavage containing drug and one gavage containing vehicle. Mice receiving drug combinations were administered two gavages containing each drug separately. B Disease progression of all recipient mice (n = 93). Peripheral blood disease progression was monitored weekly via flow cytometry and is reported as %CD5 + CD19 + of CD45 + cells. C Kaplan–Meier estimated survival of recipient mice engrafted with splenocytes from a single Eµ-TCL1 donor with BCR-dependent disease (n = 48). A log rank test was used to assess the primary statistical comparisons (vehicle vs. nemtabrutinib, vehicle vs. nemtabrutinib and venetoclax, nemtabrutinib vs. nemtabrutinib and venetoclax, and nemtabrutinib and venetoclax vs. ibrutinib and venetoclax). Holm’s method was used to adjust p values to correct for multiple comparisons (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001)