Figure 2. Biphasic regulation of severing by polyglutamylation on the β-tubulin tail by TTLL7.
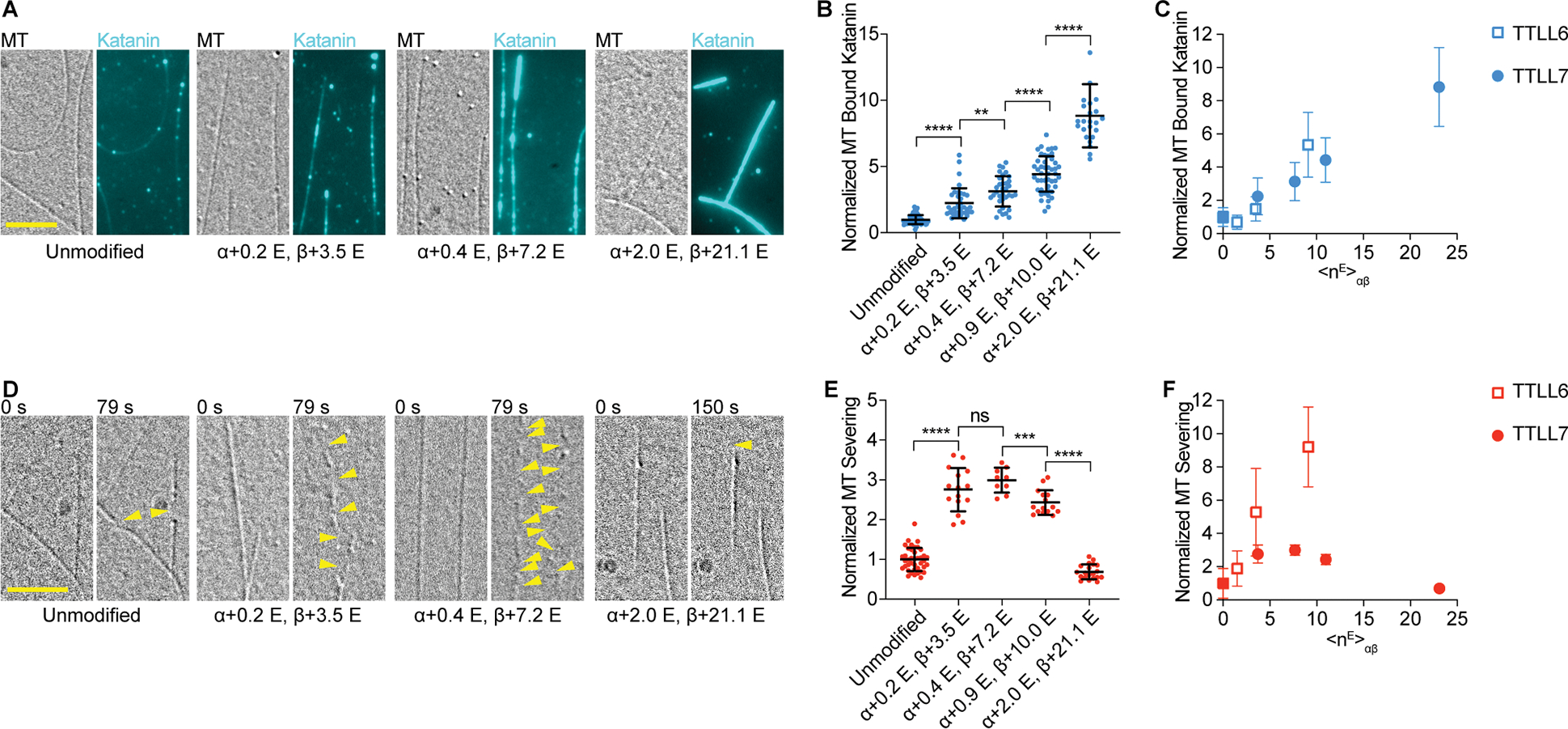
(A) Katanin association with unmodified and glutamylated microtubules with increasing glutamylation levels added by TTLL7. Assays at 4 nM katanin, 1 mM ATP; Scale bar, 5 μm.
(B) Katanin recruitment to microtubules increases with TTLL7-catalyzed glutamylation levels. Katanin levels normalized to those on unmodified microtubules; n= 67, 37, 34, 47, 24 microtubules from multiple chambers for unmodified and <nE>β ~ 3.5, 7.2, 10.0 and 21.1, respectively. Note: one data point of 16.51 for binding to <nE>β ~ 21.1 is above the y axis limit.
(C) Normalized katanin levels bound to microtubules as a function of glutamates added on tubulin by TTLL6 (open squares) and TTLL7 (filled circles); <nE>αβ, weighted average of glutamates on tubulin.
(D) Severing of unmodified microtubules and microtubules with increasing glutamylation levels introduced by TTLL7. Reactions at 20 nM katanin, 1 mM ATP; Yellow arrows, severing. Scale bar, 5 μm.
(E) Severing varies biphasically with TTLL7-catalyzed glutamylation levels. Severing rates normalized to that of unmodified microtubules; n= 40, 15, 9, 15, 20 microtubules from multiple chambers for unmodified and <nE>β ~ 3.5, 7.2, 10.0 and 21.1, respectively.
(F) Normalized severing as a function of glutamates added on tubulin by TTLL6 (open squares) and TTLL7 (filled circles). Means and S.D., p-value > 0.05 (ns), ≤ 0.01 (**), 0.001 (***), 0.0001 (****) by 2-tailed t-test. LC-MS of microtubules and additional data in Figure S2.
