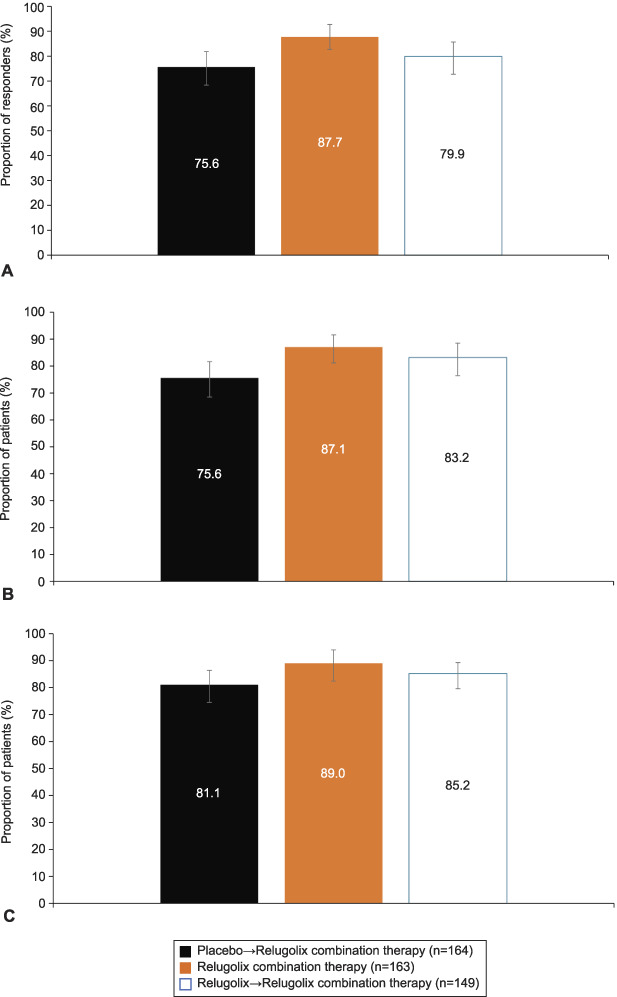
Fig. 1. A. Primary efficacy analysis results: menstrual blood loss responder rate. Treatment responder: proportion of women who achieved or maintained a menstrual blood loss volume of less than 80 mL and a 50% or greater reduction from pivotal study baseline to the last 35 days of treatment in menstrual blood loss volume. B. Proportion of patients with menstrual blood loss volume less than 80 mL over the last 35 days of treatment. C. Patients with menstrual blood loss reduction of 50% or greater. Error bars represent 95% CIs. Data shown by randomization treatment assignment.
Al-Hendy. Long-term Relugolix in Uterine Leiomyomas. Obstet Gynecol 2022.
