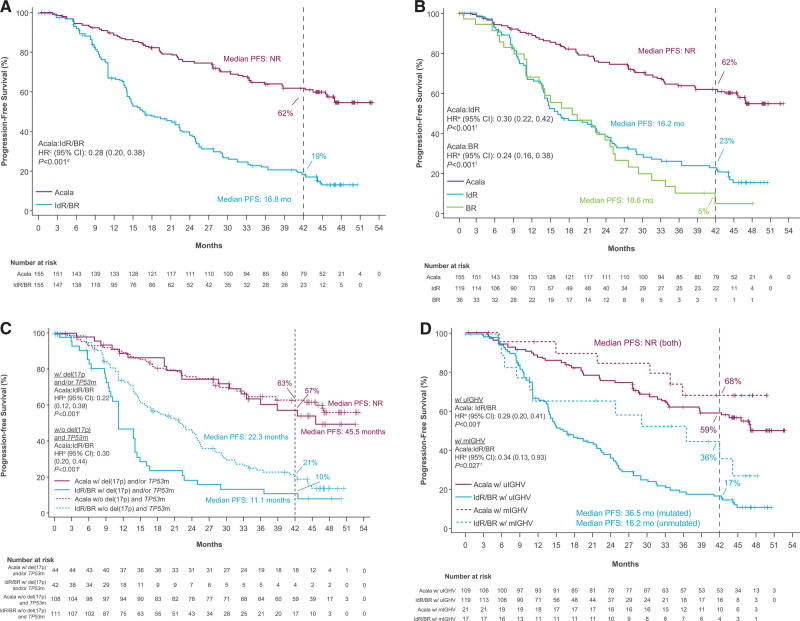
Figure 1.
Investigator-assessed progression-free survival. Outcomes are shown for (A) acalabrutinib vs IdR/BR, (B) acalabrutinib vs IdR or BR, (C) acalabrutinib vs IdR/BR by del(17p) and/or TP53 mutation status,a,b and (D) acalabrutinib vs IdR/BR by IGHV mutation status (ITT population). aMutation status for patients with del(17p) were based on values entered manually into IXRS. bBecause there were no IdR/BR–treated patients at risk by 42 mo in the del(17p) subgroup based on IXRS data, 42-mo PFS rates were not available for that analysis. cHR was based on stratified Cox proportional-hazards model, stratified by randomization stratification factors as recorded in an interactive voice/web response system. dP value was based on stratified log-rank test, stratified by randomization stratification factors as recorded in an interactive voice/web response system. eHRs were based on unstratified Cox proportional-hazards model. fP values were based on unstratified log-rank test. BR = bendamustine plus rituximab; CI = confidence interval; IdR = idelalisib plus rituximab; IGHV = immunoglobulin heavy chain variable region genes; HR = hazard ratio; ITT = intent-to-treat; IXRS = interactive voice/web response system; NR = not reached; mo, months; PFS = progression-free survival; mIGHV = mutated IGHV; TP53m, mutated tumor protein p53; TP53, tumor protein p53; uIGHV = unmutated IGHV.